The Need for An Ideal Corticosteroid
ICS are recommended as the first line anti-inflammatory therapy for the treatment of persistent asthma in children and adults and are so far the most effective controller drugs for patients with asthma.1 The chronic nature of the disease requires the need for regular treatment with often high doses of ICS over a long period. Ironically, ICS are generally underused due to lack of compliance, fear of steroids, and concerns about systemic side effects.2
ICS of the first generation had the limitation of a low therapeutic index and hence the potential for increased side effects.2 Attempts have been made to refine the topically active first generation inhaled steroids in order to improve the therapeutic index.3 This led to the formulation of second-generation inhaled corticosteroids having properties of lower systemic bioavailability and hence a superior therapeutic ratio, when compared to the older first generation corticosteroids. However, conventional second generation ICSs have shown evidence of dose-related systemic bioactivity such as hypothalamic-pituitary-adrenal (HPA) axis suppression.4,5 An increasing concern among both prescribers and patients is that, in susceptible individuals, higher doses of conventional ICSs have been associated with a clinically relevant degree of adrenal insufficiency and growth retardation.6,7
Asthma guidelines have also recognized the concern of systemic safety with long term ICS therapy, and now advocates the use of lower doses of inhaled corticosteroids, particularly when used with adjunctive second-line controller therapy.3 The dilemma in everyday practice is how to identify such at-risk individuals without subjecting all patients to time-consuming and expensive screening tests of HPA axis activity.
Additionally, in chronic diseases like asthma, adherence to treatment is a major concern for patients.8 Previous studies have shown significant improvements in adherence to treatment with once-daily compared with twice-daily controller medications.9
This brings us to the need for developing a newer generation of ICS with improved therapeutic index, particularly at higher dosages; having less frequent dosing intervals to encourage patient adherence; and maintaining clinical effectiveness and potency.10 Ciclesonide is a newer generation ICS with superior therapeutic index when compared to the older generations of ICSs, with optimum efficacy and simplicity of dosing.
Ciclesonide: The Ideal Corticosteroid
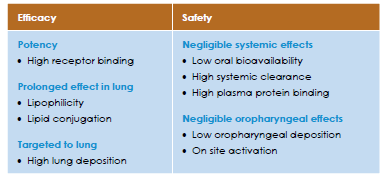
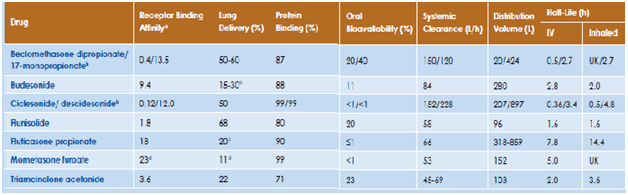
ICS are the treatment of choice for persistent asthma because of their ability to interfere with the multiple pathways involved in the inflammatory process.11 An ideal ICS should provide high local activity, such as strong receptor binding, without compromising systemic safety concerns. The characteristics of an ideal corticosteroid is tabulated in Table 1.
Ciclesonide
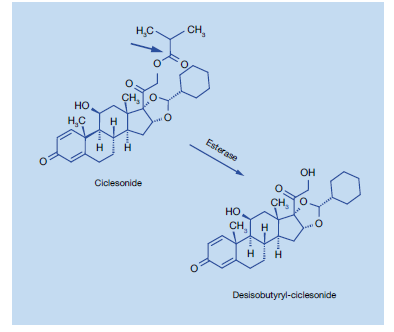
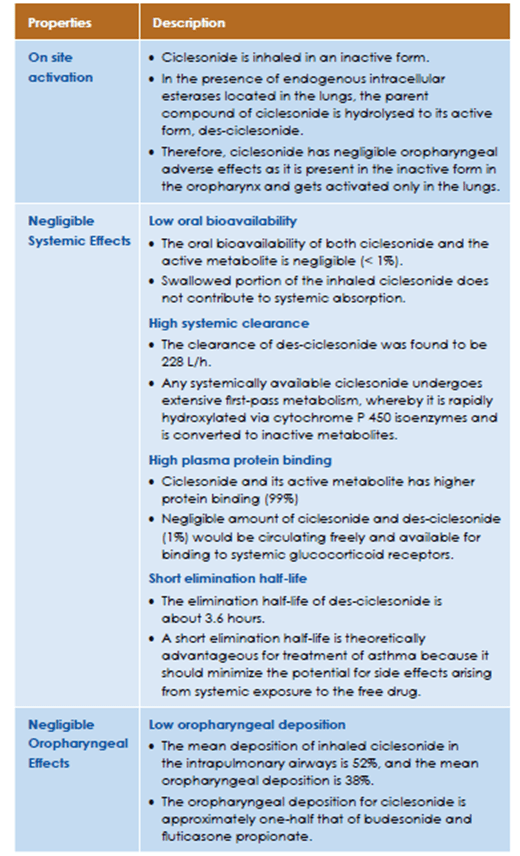
Ciclesonide is an inhaled, non-halogenated glucocorticoid with high local anti-inflammatory properties.11 It is an ester prodrug, essentially devoid of oral bioavailability, which is hydrolysed enzymatically by esterases to its active metabolite, des-ciclesonide in the lungs (Figure 1).
Properties of Ciclesonide
Several properties of ciclesonide, including its prodrug structure, high lipid affinity, and glucocorticoid receptor–binding affinity of the active drug des-ciclesonide, low oral deposition, low oral bioavailability, extensive peripheral distribution in the lung, high protein binding, and extensive first-pass metabolism, favour higher therapeutic efficacy and limited systemic exposure.13,14 The properties of ciclesonide that contributes to it’s efficacy and safety are tabulated in Table 2.
Properties of Ciclesonide that contribute to Safety:
Properties of Ciclesonide that Contribute to Efficacy:
Pharmacokinetic Properties15
Ciclesonide is formulated in HFA-134a propellant and ethanol as a solution aerosol, which demonstrates a linear relationship between different doses, puff strengths and systemic exposure.
Absorption:
Studies with oral and intravenous dosing of radiolabeled ciclesonide have shown an incomplete extent of oral absorption (24.5%). The oral bioavailability of both ciclesonide and the active metabolite is negligible (<0.5% for ciclesonide, <1%for the metabolite). Based on a γ-scintigraphy experiment, lung deposition in healthy subjects was found to be 52%. In line with this figure, the systemic bioavailability for the active metabolite is >50% by using the ciclesonide metered dose inhaler. As the oral bioavailability for the active metabolite is <1%, the swallowed portion of the inhaled ciclesonide does not contribute to systemic absorption.
Distribution:
Following intravenous administration to healthy subjects, the initial distribution phase for ciclesonide was rapid and consistent with its high lipophilicity. The volume of distribution averaged 2.9 l/kg. The total serum clearance of ciclesonide is high (average 2.0 l/h/kg) indicating a high hepatic extraction. The percentage of ciclesonide bound to human plasma proteins averaged 99%, and that of the active metabolite 98-99%, indicating an almost complete binding of circulating ciclesonide/active metabolite to plasma proteins.
Metabolism:
Ciclesonide is primarily hydrolysed to its biologically active metabolite by esterase enzymes in the lung. Investigation of the enzymology of further metabolism by human liver microsomes showed that this compound is mainly metabolized to hydroxylated inactive metabolites by CYP3A4 catalysis. Furthermore, reversible lipophilic fatty acid ester conjugates of the active metabolite were detected in the lung.
Excretion:
Ciclesonide is predominantly excreted via the faeces (67%), after oral and intravenous administration, indicating that excretion via the bile is the major route of elimination.
Pharmacodynamic Properties15
Ciclesonide exhibits low binding affinity to the glucocorticoid-receptor. Once orally inhaled, ciclesonide is enzymatically converted in the lungs to the principal metabolite (C21-des-methylpropionyl-ciclesonide) which has a pronounced anti-inflammatory activity and is considered as the active metabolite.
- In four clinical trials, ciclesonide has been shown to reduce airway hyperresponsiveness to adenosine monophosphate in hyperreactive patients with maximal effect observed at the dose of 640 micrograms.
- In another trial, pretreatment with ciclesonide for seven days significantly attenuated the early and late phase reactions following inhaled allergen challenge. Inhaled ciclesonide treatment was also shown to attenuate the increase in inflammatory cells (total eosinophils) and inflammatory mediators in induced sputum.
- A controlled study compared 24-hour plasma cortisol AUC in 26 adult asthmatic patients following 7 days of treatment. Compared to placebo, treatment with ciclesonide 320, 640, and 1,280 micrograms/day did not significantly lower the 24-hour time averages of plasma cortisol (AUC(0-24)/24 hours) nor was a dose-dependent effect seen.
- In a clinical trial involving 164 adult male and female asthmatic patients, ciclesonide was given at doses of 320 micrograms or 640 micrograms/day over 12 weeks. After stimulation with cosyntropin (1mcg and 25mcg), no significant changes in plasma cortisol levels were observed versus placebo.
- Treatment-related adverse events were seen in 3.8% and 5% of patients treated with 160 and 640 micrograms per day of ciclesonide, respectively.
Comparison of Pharmacological Properties of Various Inhaled Corticosteroids
The pharmacodynamic characteristics (glucocorticoid receptor binding) and lung delivery determine the relative clinical efficacy and pharmacokinetic properties (oral bioavailability, lung retention, systemic clearance) determine the comparative therapeutic index of various inhaled corticosteroids.16 Table 3 lists the differences among various inhaled corticosteroids in the pharmacodynamic and pharmacokinetic properties that can potentially have an impact on the therapeutic indices.
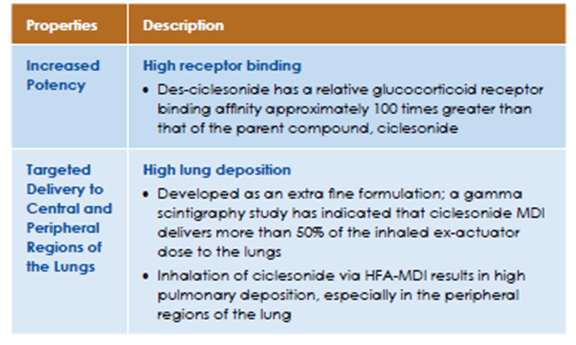
Thus, ciclesonide has low glucocorticoid receptor activity, and is activated by esterase activity in the lungs to form its active moiety, des-isobutyryl-ciclesonide (des-ciclesonide), which exhibits high glucocorticoid receptor activity, with a relative binding affinity between budesonide and fluticasone. The oral bioavailability is <1, which contributes to a higher safety margin. Des-ciclesonide is highly lipophilic, which along with formation of intracellular fatty acid conjugates results in prolonged lung tissue retention, making it suitable for once-daily dosing.
Small Airways Disease & the Need for Extrafine Formulation
The last three decades have seen important advances in the management of patients with asthma and these include the following: awareness and more timely diagnosis of the disease, pharmacological interventions targeted at controlling the underlying airways inflammation, appropriate management of the significant comorbidities associated with the condition, and a multidisciplinary approach to care for this chronic disease in the community.17 However, despite these significant advances, the present day control of disease in patients with asthma remains rather unsatisfactory.18
A key contributory factor to poor disease control might be that such patients express a ‘small airways phenotype’, where there is ongoing and unopposed small airways inflammation and dysfunction that is not being targeted or controlled by current therapies.17 To understand this better, let’s have a look at what the ‘small airways phenotype’ refers to and how it contributes to the disease progression.
What are small airways?
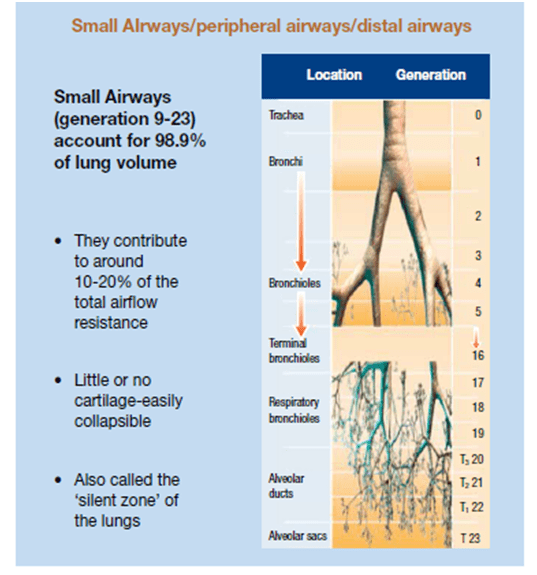
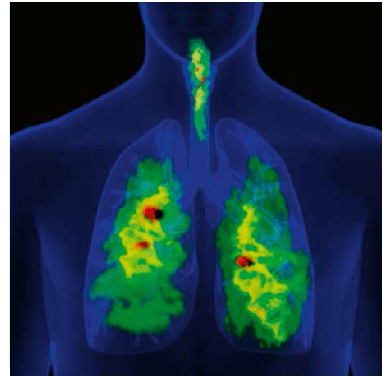
The small airways of the lungs are defined as the bronchial passages <2 mm in diameter and located beyond the seventh or eighth generation of the tracheobronchial tree.19 These airways account for >98% of the cross-sectional area of the lungs and terminate with the alveolar sacs. These small airways have no cartilage to support their structure and are, therefore, more easily collapsed upon compression. Small airways <2 mm in diameter significantly contribute to the pathogenesis of asthma, both in bronchoconstriction and hyper-responsiveness (Figure 2). The outer wall of the small airways has been recently recognised as the major site of remodelling in patients with fatal asthma.
According to a recent meta-analysis, the prevalence of small airways disease in asthma across all severities, as evaluated by impulse oscillometry, spirometry, body plethysmography, multiple-breath nitrogen washout, and high-resolution computed tomography, has been found to be 50–60%. 20 Another study has shown that small airways disease was found in more than half of the patients suffering from asthma, indicating that the routinely used lung function tests like spirometry (FEV1, FVC) and peak expiratory flow rate (PEFR), can underestimate dysfunction occurring in the small airways.21 There is now a considerable amount of evidence supporting the concept that a higher level of small airways disease is associated with increased asthma symptoms, worse asthma control, more severe bronchial hyper-responsiveness, and an increased number of exacerbations.
Medium or high doses of ICS are an option for Step 4 and step 5 of the Global Initiative for Asthma (GINA 2019) strategy and are recommended.22 However, increasing the dose of ICS does not always result in increased efficacy.23 While traditional formulations of inhaled steroids settle predominantly in the large airways, newer formulations with an extra-fine particle size have a more peripheral pattern of deposition.24 Specifically treating distal airway inflammation may improve asthma control. The following section talks about the importance of particle size on lung deposition.
Impact of Particle Size on Lung Deposition
Regional oropharyngeal and lung deposition of the inhaled particles depends on many factors, including the type and output velocity of the inhaler device, inhalation technique and airway geometry of the patient, and aerodynamic behaviour of the particles.25 One factor of key importance is the particle size.
While traditional formulations of inhaled steroids settle predominantly in the large airways, newer formulations with an extra-fine particle size have a more peripheral pattern of deposition.24 Specifically treating distal airway inflammation may improve asthma control.
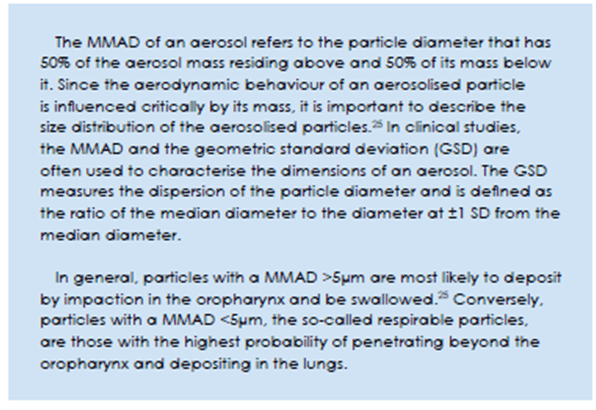
Pulmonary distribution of the medication is impacted by the size of the inhaled particle, measured in terms of the mass median aerodynamic diameter (MMAD).25
CFC pressurized metered dose inhalers with their relatively large particle size, typically delivered about 90% of their particles to the oropharynx; in contrast, approximately 50–60% of drug particles enter the lungs with HFA formulations in solution form.26
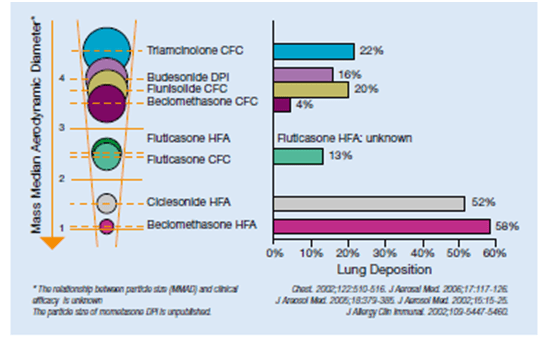
The lung deposition of various ICS is depicted in Figure 3.
This was shown in a study that examined the deposition and pharmacokinetics of ciclesonide in solution formulation administered via HFA-MDI in patients with asthma.27
Twelve patients with mild asthma (FEV1, 95% predicted) inhaled a single dose of 99mtechnetium (Tc)-ciclesonide 320 mcg.27 Deposition of ciclesonide in the lung and oropharynx was quantified using two-dimensional (2D)-gamma scintigraphy. 2D gamma scintigraphy and three-dimensional single photon emission computed tomography (3D SPECT) was used to assess the regional distribution of ciclesonide in the lung.
2D-gamma scintigraphy indicated that ciclesonide deposition was higher in the whole lung (52%) than in the oropharynx (32.9%). Furthermore, 3D SPECT revealed that ciclesonide deposition within the lungs was highest in the peripheral regions that contain the small airways and alveoli (Figure 4).
Thus, inhalation of ciclesonide via HFA-MDI results in high pulmonary deposition, especially in the peripheral regions of the lung. This may contribute to ciclesonide's ability to maintain lung function and control symptoms in patients with asthma. The deposition and activation of ciclesonide in the oropharynx is low resulting in a reduced incidence of local side effects in patients receiving ciclesonide therapy.
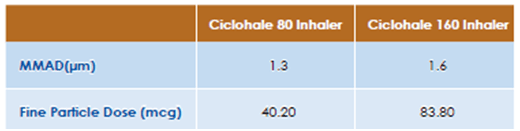
Results of an in-vitro study conducted on Ciclohale Inhaler (Ciclesonide pMDI, Cipla Ltd) evaluating its particle size distribution by Cascade Impaction is shown in Table 4 below:
Clinical Efficacy
Ciclesonide is known to have high receptor binding affinity, prolonged duration of action owing to its property of lipophilicity and increased lipid conjugation, and ensures adequate lung deposition.11 The availability of ciclesonide in an extra fine formulation ensures adequate deposition of the drug both in the central and peripheral airways.27 In clinical trials, ciclesonide in comparison with fluticasone propionate and budesonide, has demonstrated to be as effective in maintaining pulmonary function and asthma control, with the advantage of once daily dosing.
Clinical Efficacy in Adults
Once daily ciclesonide delivered via pMDI is effective and safe for the treatment of mild-to-moderate persistent asthma- An Indian Study28
-Virendra Singh et al
This was anopen label, non-comparative, multicenter study of 6 weeks, conducted in 100 subjects with mild to moderate persistent asthma. Subjects of age > 18 years, steroid naïve subjects or those who had not received inhaled steroids for at least 3 weeks, with a history of bronchial asthma for at least 3 months were included in the study. After the run-in period, all subjects were treated with inhaled ciclesonide 160 mcg (2 puffs of 80 mcg, manufactured by Cipla Ltd., India) once daily in the evening for 6 weeks. The primary efficacy variable was change from baseline in forced expiratory volume in 1 second (FEV1) and the secondary efficacy variables were change from baseline in morning peak expiratory flow rate (PEFR measured by spirometry), symptom scores and use of rescue medication.
Results:
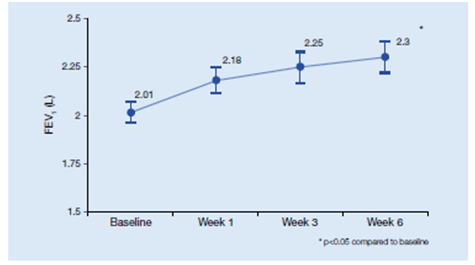
- There was a significant improvement in FEV1 at the end of 6 weeks (Figure 5)
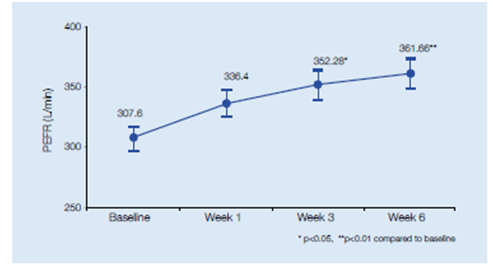
- PEFR showed significant improvement after 3 weeks of treatment (Figure 6)
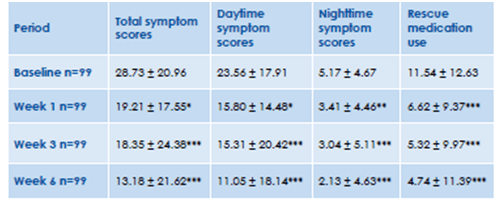
- There was a significant improvement in the symptom scores and use of rescue salbutamol after 1 week of treatment with inhaled ciclesonide (Figure 7)
- Throughout the study period, there was no change in the blood pressure, pulse and respiratory rate.
- Hematological and biochemical parameters were also unchanged.
- There were no significant differences in the urinary cortisol levels (8.00 am sample) corrected for creatinine (mcg/mg) from baseline to 6 weeks.
Thus, the study showed that ciclesonide 160 mcg HFA pMDI is efficacious and safe for the treatment of mild-to-moderate asthma.
Ciclesonide delivered as a small-particle inhaled corticosteroid improves lung function and airway hyper-responsiveness.29
-J Cohen et al
This study conducted by Cohen et al, assessed whether ciclesonide can specifically improve small airway function in asthma. A total of 16 mild-to-moderate asthma patients (19–56 yrs) were randomised to 5 weeks’ treatment with placebo (n=7) or 320 mcg ciclesonide (n=9)once daily. The following small airway parameters were assessed: mean forced expiratory flow between 25 and 75% of (FVC), percentage fall in FVC at provocative dose of adenosine-59-monophosphate and of methacholine (MCh) causing a 20% fall in FEV1, expiratory lung volume on computed tomography (CT) scan after MCh challenge, single-breath nitrogen closing volume and alveolar exhaled nitric oxide (eNO).
Results:
- Median FEF25–75% % pred increased in the ciclesonide-treated group from 52% at baseline to 63% post treatment, which was of borderline significance (p<0.051).
- The mean increase in log2PC20 of Mch was significantly larger with ciclesonide than with placebo, 1.4 versus 0.2 doubling doses, respectively (p<0.031).
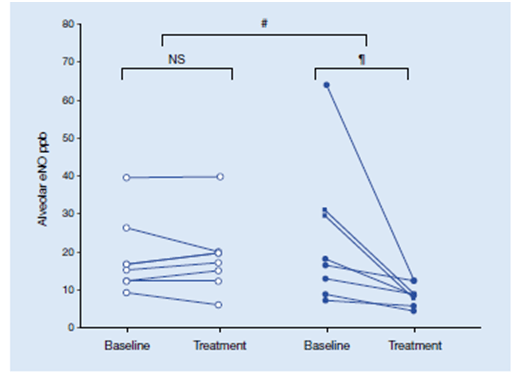
- Both alveolar eNO and CT measurements of expiratory lung volume after Mch challenge decreased significantly with ciclesonide [median (range) decrease 4.4 (54.8–1.4)ppb and 59 (1,569– -117) mL, respectively], and compared with placebo [-0.4 (7.3– -3.4) ppb and -121 (20– -236) mL respectively] (Figure 8).
- The changes in FEV1 % pred, FVC % pred and SVC % pred were also significantly larger with ciclesonide (median increase of 6, 6 and 4% pred, respectively) than with placebo (median decrease of 2, 2 and 1% pred, and p<0.003, p<0.003 and p<0.023, respectively). Bronchial median (range) eNO decreased significantly with ciclesonide (1.4 (0.2–5.6) nL/s; p<0.016), significantly more than with placebo (p<0.004)
Thus, ciclesonide improves inflammation and patency of small airways, reflected by alveolar exhaled nitric oxide and air trapping on computed tomography scan. This indicates that ciclesonide exerts anti-inflammatory effects on small airways.
Efficacy and safety profile of ciclesonide in the treatment of asthma evaluated in a large patient population in a real-life setting in Germany.30
-Claus F. Vogelmeier a, Thomas Hering b, Thomas Lewin c, Peter Sander c, Thomas D. Bethke
A real world observational study assessed 24,037 patients with persistent mild/moderate bronchial asthma. Patients received ciclesonide (160 mcg/day) and were observed for 3 months. FEV1 , PEF, NO, asthma episodes, use of rescue medication and adverse drug reactions (ADR) were recorded.
Results:
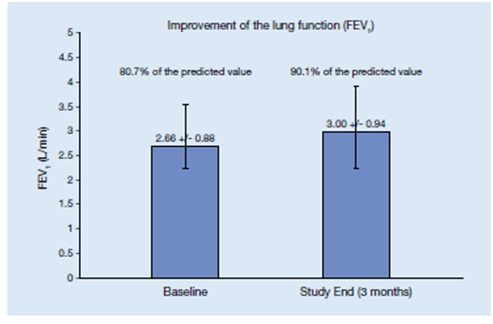
- Mean (95% CI) FEV1 significantly increased from 80.7 % of predicted at baseline to 90 % after 3 months (n =20,297) (Figure 9).
- Mean PEF significantly increased from 338 l/min to 392 l/min (n = 8100).
- NO was significantly reduced from 53.6 ppb to 26.2 ppb (n =971).
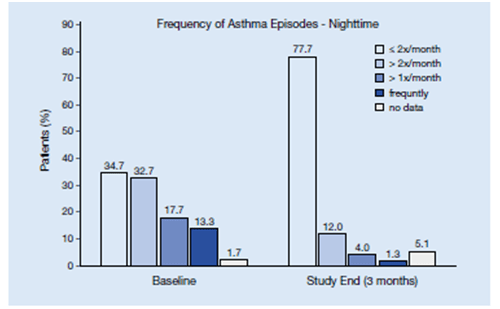
- The percentage of patients with daily symptoms declined from 24.3% to 1.9%, night-time symptoms from 13.3% to 1.3%, and beta-2 agonist use from 26.9% to 8.8%. (Figure 10)
- Most frequent adverse drug reactions were: dysphonia (n = 11), cough (n = 10), dyspnoea, throat irritation, and oral candidiasis (n = 5 each).
The analysis of this study in a large patient population of more than 24,000 patients with mild to moderate bronchial asthma concluded that ciclesonide is efficacious and well tolerated in asthmatic patients.
The efficacy profile of ciclesonide in patients with severe persistent asthma over a 1-year period.
- Brian J O’Connor et al
Patients aged 18-75 years with persistent asthma were enrolled in a 12-week, double-blind, randomized study. Patients were treated with ciclesonide 320 or 640 μg twice daily (b.i.d.) with the option of continuing in a 40-week extension phase (EP). Male and female subjects aged 18 -- 75 years with a diagnosis of persistent asthma, were eligible for inclusion in the study. Subjects had to have been treated for 4 weeks with a constant dose of 800-2000 μg/day beclomethasone dipropionate (BDP) or equivalent prior to a 2-week run-in period. Main outcomes measures were change in morning PEF from baseline to 12 weeks and safety over 1 year.
365 patients were randomized and 275 continued into the EP.
Results:
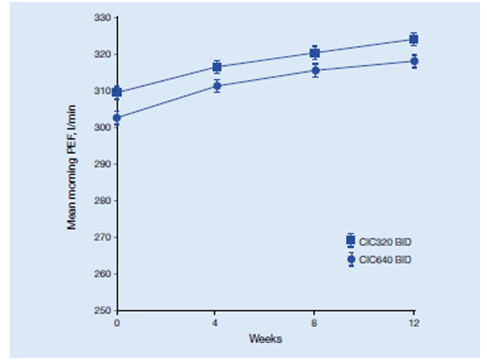
- During 12 weeks’ treatment, morning PEF significantly increased by 16 l/min (p < 0.001) and 14 l/min (p = 0.001) in the 320 and 640 μg b.i.d. groups, respectively (Figure 11) below.
- Both doses significantly reduced total asthma symptom scores by 0.29 (p < 0.0001).
- In both groups, the incidence of adverse effects (AEs) was low and mean cortisol levels in serum and urine were not suppressed during the EP.
The study concluded that Ciclesonide 320 μg b.i.d. sustained lung function and asthma symptoms in patients with severe asthma over 12 weeks’ treatment, and maintained lung function during a 40-week EP whereas ciclesonide 640 μg b.i.d. did not provide additional benefits. Thus, ciclesonide administered over a 1-year period to patients previously treated with high-dose ICSs (BDP 1600 μg/day), provided effective asthma control and a good tolerability and safety profile.
Evaluation of Efficacy and safety of once-daily ciclesonide versus twice-daily fluticasone propionate at comparable daily doses in patients with asthma.32
-Rohland Buhl et al
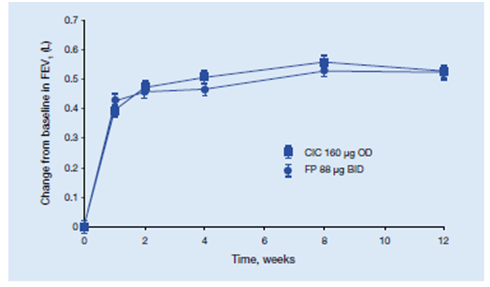
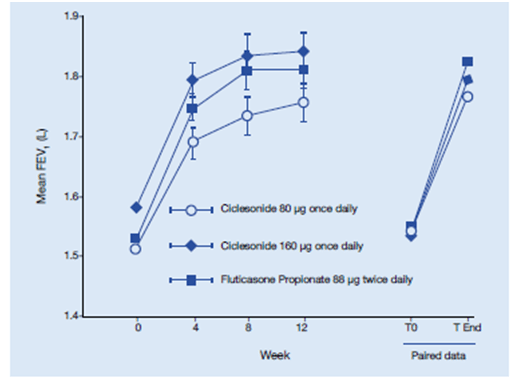
This study was a multicentre, randomized, double-blind, double-dummy, parallel group study in which 529 patients with asthma were randomized to ciclesonide 160 mg once daily or fluticasone propionate 88 mcg twice daily for 12 weeks. The primary endpoint was change in lung function.
Results:
- Both ciclesonide and fluticasone propionate significantly FEV1, FVC and PEF compared with baseline. (Figure 12)
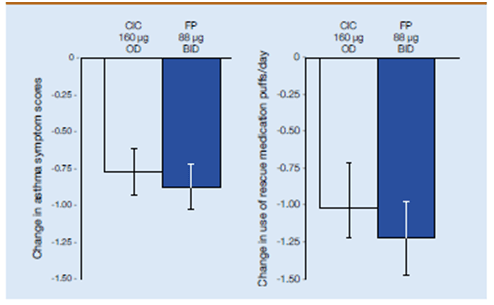
- Both medications reduced asthma symptoms and rescue medication use within the first 24 h. At the tested dose, both medications were equally safe and well tolerated.
- Night time asthma symptom scores improved by -0.29 and -0.33 for ciclesonide and fluticasone propionate, respectively, whereas daytime asthma symptom scores improved by -0.43 in the ciclesonide group and -0.50 in the fluticasone propionate group (p<0.0001 versus baseline for both; ITT analysis) given in Figure 13.
- Ciclesonide and fluticasone propionate also significantly reduced rescue medication use (p<0.0001 versus baseline for both treatments; Figure 13) with no significant differences between the groups
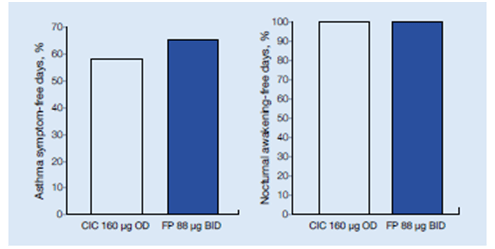
Comparable increases in the median percentage of days without asthma symptoms and without nocturnal awakenings were observed in both treatment groups (Figure 14).
The improvements in symptom score, rescue medication use and lung function demonstrated in this study, were achieved with once-daily dosing of ciclesonide versus twice-daily dosing of fluticasone propionate at similar daily doses.
Comparison of the efficacy of ciclesonide with that of budesonide in mild to moderate asthma patients after step-down therapy: a randomised parallel-group study33
Kuo-Chin Chiu et al
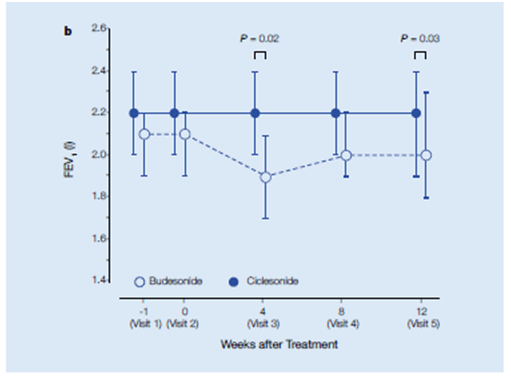
This study aimed to compare the clinical benefit of ciclesonide with budesonide (BUD) as step-down therapy. This is one of the first studies that compares a step-down therapy strategy between mono-ICSs from a combination therapy with ICSs and LABAs in patients with well controlled mild to moderate asthma. A total of 150 patients with mild-to-moderate asthma well controlled by a combination of ICS and LABA for atleast 3 months, were randomised to receive either ciclesonide 320 μg (n = 75) once daily or 2 inhalations of BUD 200 μg (n = 75) twice daily for 12 weeks. Primary efficacy variable was the improvement in FEV1 at the end of the treatment period (12 weeks). Secondary efficacy parameters included the measurements of (FVC) and maximum mid-expiratory flow (MMEF) through the treatment period.
Results:
Improvement in FEV1.
- The FEV1 remained stable throughout the 12-week ciclesonide treatment.
- In the BUD group, FEV1 significantly decreased at weeks 4 and 12 (Figure 15).
- The FEV1 (before bronchodilators) of the ciclesonide group (2.2 l, 95% CI, 2.0–2.4 lL, n = 72) was significantly higher than that of the budesonide group (1.9 l, 95% CI, 1.7–2.1 l, n = 65, P = 0.02) at 4 weeks and at the end of 12 weeks (2.0 l, 95% CI, 1.8–2.3 l, n = 44, P = 0.03) of step-down therapy.
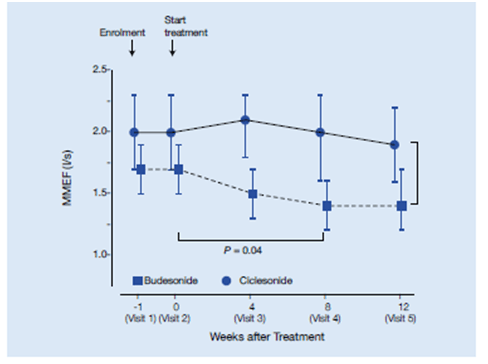
Improvement in MMEF:
- The MMEF in the CIC group was significantly higher than that of the BUD group (P = 0.02), especially after 4 and 8 weeks (CIC, 2.0 l/s, 95% CI, 2.3–1.6 l/s, n = 61 vs. BUD, 1.4 l/s, 95% CI, 1.6–1.2 l/s, n = 53, P = 0.04) (Figure 16).
- Drug adherence was significantly higher in the ciclesonide group than in the budesonide group (76.0% vs. 58.7%, P = 0.03).
This study shows the beneficial effects of extra fine ciclesonide formulation on both large airway parameter (FEV1) and small airway parameter as demonstrated by MMEF (a parameter used to detect small airways inflammation). There was greater improvement in MMEF with ciclesonide compared to budesonide from baseline up to 8 weeks of the study period. The adherence to treatment was also significantly higher in the ciclesonide group compared to the budesonide group, thus, highlighting the advantages of ciclesonide with respect to efficacy and adherence.
Clinical Efficacy in Children
Once-daily ciclesonide in children: efficacy and safety in asthma34
-Gelfand EW
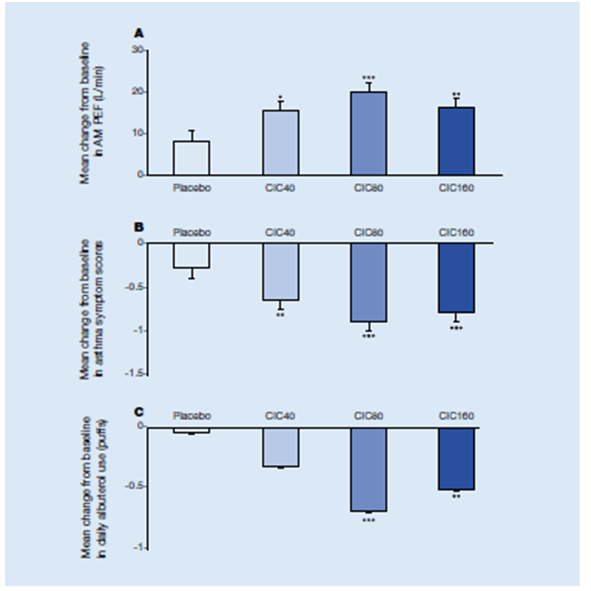
- In this double-blind, placebo-controlled, parallel group study, 1031 children with asthma (age 4 to 11 years) were randomized to receive either once-daily inhaled ciclesonide 40 mcg (CIC40), 80 mcg (CIC 80), 160 mcg (CIC 160) or placebo. Baseline characteristics were comparable; 59.4% of patients had moderate asthma, and 24.1% had severe asthma.
- All ciclesonide doses were associated with greater improvements in baseline to week 12 FEV1 percent predicted versus placebo (CIC40, 11.97; CIC80, 13.58, P <.05; CIC160, 14.17, P < .01). Significant improvements in asthma symptoms (P < .01) and reductions in salbutamol use were reported (Figure 17).
- All ciclesonide doses were associated with a statistically significant improvement in the overall Paediatric Asthma quality of Life Questionnaire (PAQLQ score versus baseline) compared with placebo (CIC40, 0.52; CIC80, 0.54; CIC160, 0.56; placebo, 0.27; P < .05 for all vs placebo).
- Ciclesonide was well tolerated with no effect on HPA axis function.
Thus, the study results demonstrated that once daily ciclesonide was effective and well tolerated in children with persistent asthma.
Efficacy and safety evaluation vs Fluticasone35
-S. Pedersen et al.
Ciclesonide was compared with fluticasone propionate (FP) in a 12-week, randomized, double-blind, double-dummy, three-arm, parallel-group study in children (6-11 years) with moderate and severe persistent asthma. Efficacy in terms of FEV1, PEFR, asthma symptom scores, rescue medication use and quality of life was evaluated in 744 children receiving ciclesonide (80 or 160 mg once daily) or fluticasone propionate (88 mg twice daily). Systemic effect was assessed by 24-hour urine free cortisol adjusted for creatinine.
Results:
- There was a significant improvement in FEV1, morning PEF (Figure 18), asthma symptom score and rescue medication use from baseline in all groups (p < 0.0001). Ciclesonide 160 mcg was found to be non inferior to fluticasone propionate 176 mcg for FEV1 (p = 0.0030, one-sided).
- The percentages of asthma symptoms, rescue medication, nocturnal awakening, symptom free days showed no significant differences between treatments.
- Quality of life scores improved with all treatments (p < 0.0001) with no significant difference between the groups (p < 0.0001, one-sided, for all).
- The incidences of adverse events were comparable across treatments. Urine free cortisol levels decreased significantly with fluticasone propionate (p = 0.0103), but not with ciclesonide (Figure 19).
Thus once-daily ciclesonide showed a clinical effect similar to that of fluticasone propionate, with no effect on cortisol suppression, in children with moderate and severe asthma.
Randomized comparison of the efficacy and safety of ciclesonide and budesonide in adolescents with severe asthma36
- J.H. Vermeulena
In this randomized, double-blind, double-dummy, parallel-group study,400 patients aged 12–17 years with severe asthma were treated with budesonide 400 mcg once daily (QD) in a 2-week run-in period. At randomization, eligible patients were assigned 2:1 to ciclesonide 320 mcg QD (ex-actuator) or budesonide 800 mcg QD (metered dose), respectively, in the evening. FEV1 was the primary efficacy variable. Patients recorded asthma symptom score and rescue medication use in diaries. Safety assessments included adverse events (AEs) and 24-h urine cortisol.
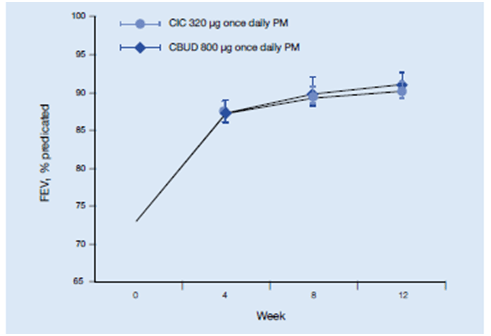
- FEV1% predicted increased in the ciclesonide group from 73.1% at baseline to 89.4% at study end, and in the budesonide group from 73.0% to 90.7% (ITT analysis; LS means); there was no significant difference between treatment groups (Figure 20).
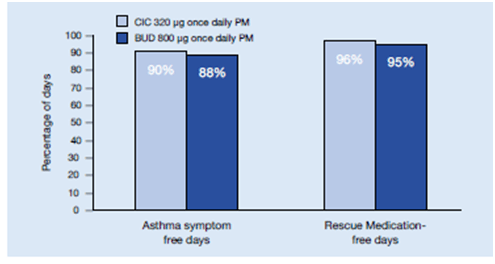
- Assessment of asthma symptom scores, as well as percentages of asthma symptom-, rescue medication and nocturnal awakening-free days, also revealed no statistically significant or clinically relevant differences between the efficacy of ciclesonide and the higher dose of budesonide (Figure 21)
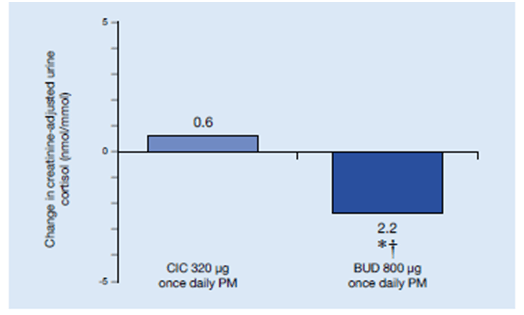
- Median urine cortisol significantly decreased in the budesonide group from baseline to study end (15.9–13.7 nmol cortisol/mmol creatinine; p=0.0086). The change in the ciclesonide group was not significant (15.9–16.5 nmol cortisol/mmol creatinine; p=0.1125) , while the difference between treatment groups was significant (p=0.0012) (Figure 22).
In this study, equipotent doses of ciclesonide and budesonide were evaluated in adolescent patients who were not well controlled on their previous treatment. All efficacy parameters remaining similar, only budesonide, but not ciclesonide, had a suppressive influence on the HPA axis, an indirect measure of systemic exposure, as indicated by a significant decrease in 24-h urine cortisol levels.
Safety
Ciclesonide is a nonhalogenated ICS, available as an HFA-MDI in two strengths, 80 mcg per actuation and 160 mcg per actuation, administered once-daily. Several properties, including its prodrug structure, high lipid affinity, and glucocorticoid receptor–binding affinity of the active drug des-ciclesonide, low oral deposition, low oral bioavailability, extensive peripheral distribution in the lung, high protein binding, and extensive first-pass metabolism, favour higher therapeutic efficacy and limited systemic exposure.
Undesirable Effects:
Ciclesonide is generally safe and well tolerated in patients.37
Clinical trial data in adults and adolescents
The safety of ciclesonide was evaluated in 24,037 patients with persistent mild/moderate bronchial asthma in Germany. 30
- During the 3 month treatment period, adverse drug reactions (ADRs) were reported in 51 (0.2%) patients, and 0.001% patients discontinued therapy, due to ADRs or unknown and missing reasons.
- Most ADRs were of mild or moderate intensity.
- Most commonly reported non-serious ADRs (n = 50) were dysphonia (n = 11) and cough (n = 10). In 1 patient, the ADR was classified as serious, with fatal outcome due to mycocardial infarction (no relation to ciclesonide as assessed by the investigator).
- In the same study, 20 children of 6-11 years were treated with ciclesonide. In these patients too, treatment with ciclesonide was well tolerated.
The safety of ciclesonide has been depicted in several studies which have assessed the systemic effects of the drug. The studies are listed below.
- HPA axis suppression:
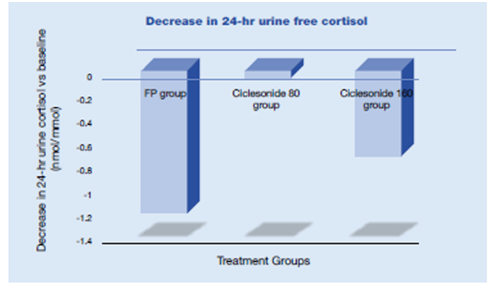
Lipworth et al evaluated the potential effects of ciclesonide therapy on the dynamic cortisol response to sequential low- and high-dose cosyntropin stimulation in adults with mild-to moderate persistent asthma in a double-blind, placebo controlled, 12-week study.38 In this study 164 patients were randomized to placebo, 320 mcg ciclesonide once-daily, 320 mcg ciclesonide twice-daily (all ciclesonide doses delivered via HFA-MDI), and 440 mcg FP twice-daily delivered via CFC-MDI. Patients had normal HPA-axis function at screening and had not used systemic corticosteroids within 6 months of screening or inhaled or intranasal corticosteroids within 2 months of screening.
- Ciclesonide did not produce any significant suppression of either basal cortisol levels or the response to cosyntropin stimulation, with results almost identical to the placebo group.
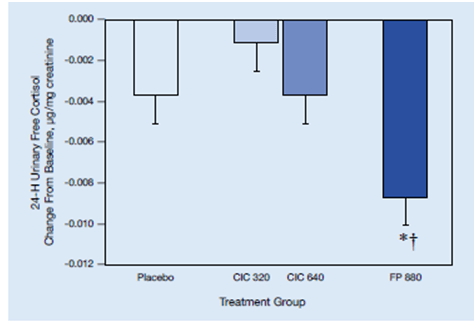
- In contrast, the FP group showed significant suppression of 24-hour-urinary-free cortisol levels and on high-dose cosyntropin stimulation compared to the placebo group (Figure 23).
- The differences between ciclesonide groups and FP were statistically significant. Thus, ciclesonide may result in less adrenal suppression than FP.
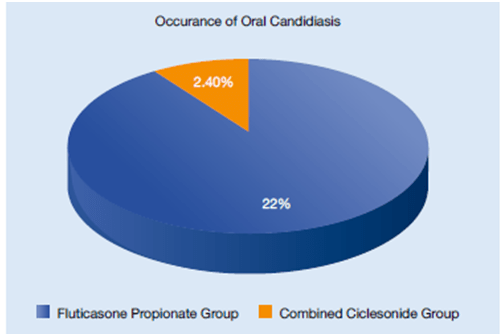
- Oral candidiasis occurred in 22% of FP group compared to 2.4% in the combined ciclesonide groups. Hoarseness occurred at the rate of 7.3% in the FP group and 2.4% in the combined ciclesonide groups (Figure 24).
- Bone metabolism
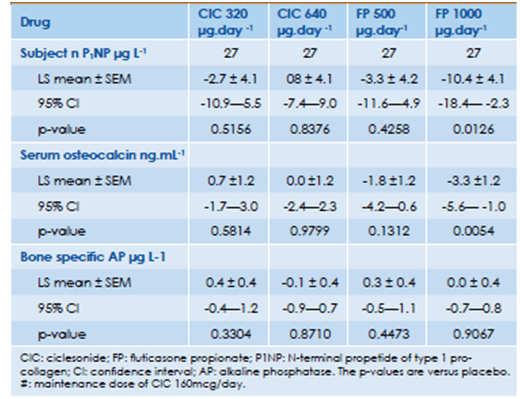
Derom et al compared the systemic and clinical effects of ciclesonide (CIC) and fluticasone propionate (FP) administered, in addition to CIC 160 mcg/day and salmeterol 50 mcg twice daily, in 32 patients with persistent asthma using a randomised double-blind, placebo-controlled, double dummy, five-period crossover design.39 Markers of bone metabolism namely; N-terminal propeptide of type 1 pro-collagen (P1NP), serum osteocalcin and alkaline phosphatase were evaluated.
- No significant differences were noted after any ciclesonide treatment compared with placebo for any bone formation marker, which included N-terminal propeptide of type 1 procollagen (P1NP), alkaline phosphatase (AP), and serum osteocalcin.
- FP 1000 mcg caused significant decreases in P1NP (P = 0.0126) and serum osteocalcin levels (P = 0.0054) compared to placebo. The clinical significance of these findings is not clear.
- Lenticular Opacity
Chylack et al demonstrated that treatment with ciclesonide for 1 year, had minimal impact on lenticular opacity development.40 Adults (>or=18 years of age) with moderate-to-severe asthma were randomized to ciclesonide 640 mcg/day (n = 785) or beclomethasone dipropionate 640 mcg /day (n = 783) in a multinational, double-blind, active-controlled, parallel-group study. The primary endpoint was the occurrence of a positive Class I grading shift (increase [worsening] in Lens Opacities Classification System [LOCS] III score of >or= 0.5 for nuclear opalescence, >or= 0.8 for cortical opacification, or >or= 0.5 for posterior subcapsular opacification, or cataract surgery) in either eye at any visit over the 12-month treatment period. Mean changes in LOCS III scores were very small in both groups, thus proving treatment with ciclesonide 640 mcg/day or beclomethasone dipropionate 640 mcgg/day for 1 year has a minimal impact on lenticular opacities development and/or progression.
- Growth Rate:
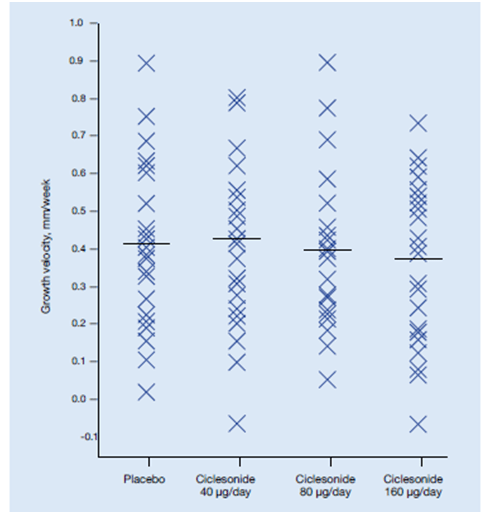
Agertoft L et al evaluated the impact of ciclesonide treatment on cortisol levels in urine in 24 children in a double-blind, placebo-controlled, 4-period crossover study.41 Children aged 6-12 years sequentially received ciclesonide (40, 80 and 160 µg) in randomised order once daily in the evening. Each 2-week treatment period was followed by a 2-week washout period. Knemometry was performed at the beginning and end of each treatment period. Cortisol levels in 12-hour overnight urine were measured at the end of each treatment period.
- No statistically significant differences were seen in lower leg growth rates between any of the ciclesonide treatments and placebo.
- Lower leg growth rates were 0.412 mm/week for placebo, 0.425 mm/week for 40 µg of ciclesonide, 0.397 mm/week for 80 µg of ciclesonide and 0.370 mm/week for 160 µg of ciclesonide. There was no statistically significant dose response effect (Figure 25).
- Likewise, no statistically significant differences of dose response effects were found for urinary cortisol adjusted for creatinine.
- Thus, short-term lower leg growth rate and hypothalamic-pituitary-adrenal axis functions are not affected by treatment with ciclesonide in doses intended for clinical use in children.
- Reversibility of adrenal suppression:
A case series described 15 children with asthma who developed adrenal suppression while receiving long-term conventional ICS therapy.42 There was decrease in linear growth velocity (GV) in these children which was related to adrenal suppression. These patients were then shifted to ciclesonide treatment.
- A mean decrease in height standard deviation score in the year prior to diagnosis of adrenal suppression was -0.59, -0.11, and -0.18, in prepuberty, peri-puberty, and post-puberty patients, respectively.
- Changing the inhaled corticosteroid to ciclesonide led to recovery of adrenal function without impacting asthma control. A change in growth velocity of +5.3cm/yr, +2.1cm/yr, and -1.9cm/yr, was noted for pre-puberty, peri-puberty, and post-puberty patients, respectively after starting ciclesonide.
- The study highlighted the importance of monitoring height in pediatric patients receiving inhaled corticosteroids which would serve as a good marker for adrenal suppression.
In all 15 cases, recovery of adrenal function was achieved after ICS therapy was switched to ciclesonide, and asthma control remained optimum.
Therefore, ciclesonide has a favourable safety and tolerability profile compared to other inhaled corticosteroids as it has negligible effects on adrenal axis suppression and systemic & local side effects.
Ciclohale Inhaler
For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only
Composition
CICLOHALE 80 Inhaler
Each actuation (delivered dose from the mouthpiece) delivers
Ciclesonide IP…………………….80 mcg
In propellant HFA 134a…………q.s.
Absolute alcohol content ………..8.8%v/v
CICLOHALE 160 Inhaler
Each actuation (delivered dose from the mouthpiece) delivers
Ciclesonide IP…………………….160 mcg
In propellant HFA 134a…………q.s.
Absolute alcohol content ………..8.8%v/v
Dosage Form and Strength
CICLOHALE 80 Inhaler
Inhalation aerosol containing 80 mcg per actuation
CICLOHALE 160 Inhaler
Inhalation aerosol containing 160 mcg per actuation
Clinical Particulars
Therapeutic Indications
CICLOHALE is indicated for treatment of persistent asthma in adults (18 years and older).
Posology and Method of Administration
CICLOHALE is for oral inhalation use only.
Symptoms start to improve with ciclesonide within 24 hours of treatment. However, due to its prophylactic nature, CICLOHALE should be taken regularly even when patients are asymptomatic.
Dosing recommendation for adults aged 18 years and older
The recommended dose range is 80 to 320 mcg once daily in adult patients. Patients should be given a starting dose of CICLOHALE which is appropriate to the severity of their disease. In patients with severe asthma and while reducing or discontinuing oral corticosteroids, a higher dose of up to 640mcg/day (given as 320mcg twice daily) may be used.
Contraindications
CICLOHALE is contraindicated in patients with history of hypersensitivity to ciclesonide or any of the components of the formulation
Special Warnings and Precautions for Use
As with all inhaled corticosteroids (ICS), CICLOHALE should be administered with caution in patients with active or quiescent pulmonary tuberculosis fungal, bacterial or viral infections, and only if these patients are adequately treated. As with all ICS, CICLOHALE is not indicated in the treatment of status asthmaticus or other acute episodes of asthma where intensive measures are required.
As with all ICS, CICLOHALE is not designed to relieve acute asthma symptoms for which an inhaled short-acting bronchodilator is required. Patients should be advised to have such rescue medication available. Patients with severe asthma are at risk of acute attacks and should have regular assessments of their asthma control including pulmonary function tests. Increasing use of short-acting bronchodilators to relieve asthma symptoms indicate deterioration of asthma control. If patients find that short-acting relief bronchodilator treatment becomes less effective, or they need more inhalations than usual, medical attention must be sought. In this situation, patients should be reassessed and consideration given to the need for increased anti-inflammatory treatment therapy (eg higher doses of ICS or a course of oral corticosteroids). The maximal daily dose is 640 μg/day (given as 320 μg twice a day but the superiority of this dose over 320 μg/day has not been unequivocally demonstrated (see Clinical trials).
Systemic Effects
Inhaled steroid products are designed to direct glucocorticoid delivery to the lungs in order to reduce overall systemic glucocorticoid exposure and side effects. In sufficient doses however, all ICS can have adverse effects, notably depression of the hypothalamic-pituitary-adrenal (HPA) axis, reduction of bone density, cataract, glaucoma and retardation of growth rate in children and adolescents. In steroid dependent patients, prior systemic steroid usage may be a contributing factor, but such effect can occur amongst patients who use only ICS regularly.
HPA axis suppression and adrenal insufficiency
The lowest dose of ciclesonide that causes suppression of the HPA axis (as indicated by 24-hour urinary cortisol concentrations), effects on bone mineral density or growth retardation in patients has not yet been established.
A controlled study compared 24-hour plasma cortisol AUC in 26 adult asthmatic patients following 7 days of treatment. Compared to placebo, treatment with ciclesonide 320, 640 and 1280 μg/day did not statistically lower the 24-hour time averages of plasma cortisol (AUC(0-24)/24 hours) nor was a dose-dependent effect seen. Hence, at therapeutic doses, no significant difference was detected between inhaled ciclesonide and placebo on HPA function and serum cortisol levels. However, potential effects on the HPA axis may occur in individual patients particularly at times of physiological stress (eg hot climate, illness or surgery). Similar results were seen in other studies in asthmatic children aged 4 to 12 years.
Growth
It is recommended that the height of children and adolescents receiving prolonged treatment with ICS is regularly monitored. In addition, consideration should be given to referring the patient to a paediatric respiratory specialist.
Visual disturbance
Visual disturbance may be reported with systemic and topical corticosteroid use. If a patient presents with symptoms such as blurred vision or other visual disturbances, the patient should be considered for referral to an ophthalmologist for evaluation of possible causes which may include cataract, glaucoma or rare diseases such as central serous chorioretinopathy (CSCR) which have been reported after use of systemic and topical corticosteroids.
Transfer from oral corticosteroids
The benefits of inhaled ciclesonide should minimise the need for oral corticosteroids. However, patients transferred from oral steroids may remain at risk of impaired adrenal reserve for a considerable time after transferring to inhaled ciclesonide. The possibility of adverse effects may persist for some time. These patients may require specialised advice to determine the extent of adrenal impairment before elective procedures. The possibility of residual impaired adrenal response should always be considered in emergency (medical or surgical) and elective situations likely to produce stress, and appropriate corticosteroid treatment considered. Transfer of patients from systemic corticosteroid therapy to CICLOHALE may unmask pre-existing allergic conditions such as allergic rhinitis or eczema, previously suppressed by systemic corticosteroid therapy.
General
Paradoxical bronchospasm with an immediate increase of wheezing or other symptoms of bronchoconstriction after dosing should be treated with an inhaled short-acting bronchodilator, which usually results in quick relief. If the patients find short-acting bronchodilator treatment ineffective, or they need more inhalations than usual, medical attention must be sought. This indicates a worsening of the underlying conditions, and warrants a reassessment of the therapy.
The patient should be assessed and therapy with CICLOHALE should only be continued, if after careful consideration the expected benefit is greater than the possible risk. Correlation between severity of asthma and general susceptibility for acute bronchial reactions should be kept in mind.
The patient should be advised against abrupt discontinuation of therapy with CICLOHALE.
Patient inhaler technique should be checked regularly to make sure that inhaler actuation is synchronized with inhalation to ensure optimum delivery to the lungs.
Concomitant treatment with ketoconazole or other potent CYP3A4 inhibitors should be avoided unless the benefit outweighs the increased risk of systemic side effects of corticosteroids.
Use in hepatic impairment
Systemic exposure to the active metabolite (M1) is increased in patients with hepatic impairment. Although no dosage reduction is necessary, prescribers should be aware of the possibility of an increased risk of systemic adverse effects.
Use in the elderly
Systemic exposure to M1 is also increased in elderly patients. Although no dosage reduction is necessary, prescribers should be aware of the possibility of an increased risk of systemic adverse effects in such patients.
Paediatric use
To date, there is insufficient data available in the treatment of children of 5 years and younger with Ciclesonide.
Effects on laboratory tests
No data available.
Drug Interactions
In a drug-drug interaction study at steady state with ciclesonide and ketoconazole as a potent CYP3A4 inhibitor, the exposure to the active metabolite M1 increased approximately 3.5-fold, whereas the exposure to ciclesonide was not affected. Therefore, the concomitant administration of potent inhibitors of CYP3A4 (eg ketoconazole, itraconazole and ritonavir or nelfinavir) should be avoided unless the benefit outweighs the increased risk of systemic side effects of corticosteroids
Use in Special Populations
Geriatrics: In a comparison between one study in elderly subjects and another study in young healthy subjects, there was an approximately 2-fold increase in the rate and extent of exposure to the active metabolite in elderly patients. However, in a population pharmacokinetic analysis of 9 studies, age did not impact the clearance or volume of distribution of the active metabolite.
Pediatrics: In two 12 week clinical studies investigating the safety and efficacy of Ciclesonide in asthmatic patients between 4-11 years of age, serum samples were taken from 53 patients for pharmacokinetic analysis. The pharmacokinetics of the active metabolite M1 were found to be similar to adults.
Hepatic Insufficiency: Reduced liver function may affect the elimination of corticosteroids. In a study including patients with hepatic impairment suffering from liver cirrhosis, a higher systemic exposure (1.8 to 2.8 times) to the active metabolite was observed.
Renal Insufficiency: Due to the low rate of renal excretion of ciclesonide metabolites, studies on renally impaired patients have not been performed.
Pregnancy: Category B3
There are no adequate and well controlled studies in pregnant women. In animal studies glucocorticoids have been shown to induce malformations. Corticosteroids are known to induce foetotoxic and teratogenic effects in rodent and rabbit studies. Embryofoetal development studies with daily SC dosing of ciclesonide in rabbits, abnormal foetal development (cleft palate, hind paw flexure, enlarged fontanelle, parchment like skin) was observed at systemic exposure levels (based on plasma AUC) ranging from about 3 to 12 times that anticipated clinically at the maximum recommended human dose. Embryofoetal development studies in rats showed reduced foetal weight, skeletal anomalies, hydronephrosis and maternotoxicity at oral doses of 300-900 μg/kg/day. Similar studies with these doses extended until weaning revealed maternotoxicity, reduced pup weight gain, changes in pup organ weight and changes in behavioural development tests. The systemic exposure of dams relative to human exposure in these studies is not known, but doses represented 2-6 times the maximum recommended human dose on a body surface area basis. As with other ICS preparations, CICLOHALE is not to be used during pregnancy unless the potential benefit to the mother justifies the potential risk to the mother or foetus. The lowest effective dose of ciclesonide needed to maintain adequate asthma control should be used. Infants born of mothers who received corticosteroids during pregnancy are to be observed carefully for hypoadrenalism.
Lactation:
The excretion of ciclesonide or its metabolites into human milk has not been investigated. There was limited excretion of ciclesonide and/or its metabolites into milk in lactating rats after intravenous or oral administration (respective maxima of 0.23% and 0.03% of dose/g tissues). Oral administration of ciclesonide to rats from early pregnancy until weaning was associated with adverse effects on pups. In breastfeeding mothers, the therapeutic benefits of the drug should be weighed against the potential hazards to mother and baby.
Effects on Ability to Drive and Use Machines
Inhaled ciclesonide has no or negligible influence on the ability to drive and use machines
Underirable Effects
Clinical trial data in adults and adolescents
Approximately 5% of patients experienced adverse reactions in clinical trials with Ciclesonide given in the dose range 80 to 1280 μg per day. In the majority of cases, these were mild and did not require discontinuation of treatment with Ciclesonide.
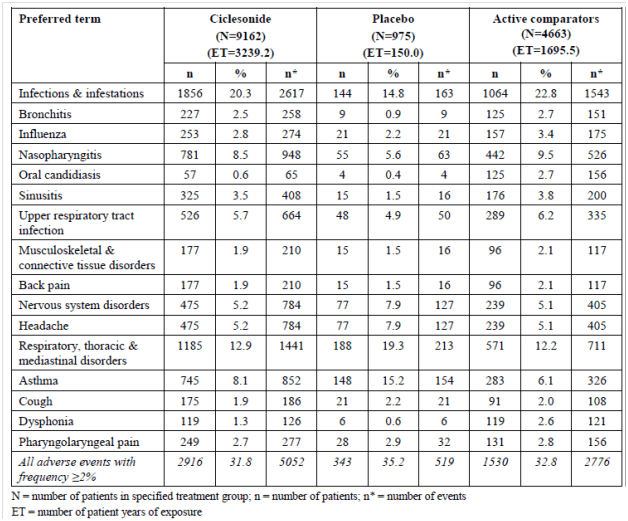
The table below shows the adverse events reported with a frequency of ≥2% from participants in studies of up to 1-year duration.
Adverse events that occurred ≥2% from participants in studies of up to 1-year duration
% = percentage of patients with specified event based on N
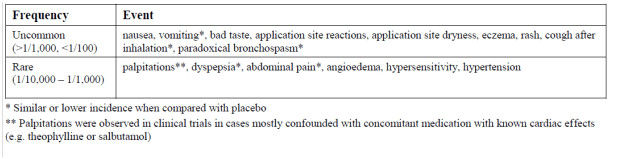
The following adverse reactions have also been reported in clinical trials with Ciclesonide:
Adverse reactions also reported in clinical trials with Ciclesonide
Paradoxical bronchospasm may occur immediately after dosing and is an unspecific acute reaction to all inhaled medications, which may be related to the drug, the excipient, or evaporation cooling in the case of metered dose inhalers. In the majority of cases, this reaction is mild and does not require withdrawal of CICLOHALE. In severe cases, withdrawal of CICLOHALE should be considered.
Post marketing experience
Very rare cases of immediate or delayed hypersensitivity reactions such as angioedema with swelling of lips, tongue and pharynx have been reported from spontaneous reporting with Ciclesonide. There have been very rare reports of psychiatric symptoms such as agitation, insomnia, depression, anxiety and behavioural changes with ciclesonide as well as with other ICS.
Systemic effects of ICS may occur, particularly at doses higher than recommended. Possible systemic effects include Cushing’s syndrome, Cushingoid features, adrenal suppression, growth retardation in children and adolescents and decrease in bone mineral density. Eye disorders with frequency unknown, such as blurred vision, cataract or glaucoma, have been reported with systemic and topical corticosteroid use.
If you experience any side-effects, talk to your doctor or pharmacist or write to drugsafety@cipla.com. You can also report side effects directly via the national pharmacovigilance program of India by calling on 1800 180 3024 or you can report to Cipla Ltd on 18002677779. By reporting side-effects, you can help provide more information on the safety of this product
Overdose
Acute
Inhalation by healthy volunteers of a single dose of 2880 mcg of ciclesonide was well tolerated. The potential for acute toxic effects following overdose of inhaled CICLOHALE is low. After acute overdosage no specific treatment is necessary.
Chronic
After prolonged administration of 1280 mcg of ciclesonide no significant clinical signs of adrenal suppression were observed. However, if higher than recommended dosage is continued over prolonged periods, some degree of adrenal suppression cannot be excluded. Monitoring of adrenal reserve may be necessary. In cases of ciclesonide overdose, therapy may still be continued at a suitable dosage for symptom control.
Pharmacological Properties
Mechanism of Action
Ciclesonide (pure R-epimer) belongs to a new class of on-site activated non-halogenated ICS. Ciclesonide is an ester pro-drug with approximately 100-fold lower affinity for the glucocorticoid receptor than its active metabolite (M1; 21-des-isobutyryl-ciclesonide) and budesonide and fluticasone. Endogenous activation occurs primarily via esterases located in the lung, to give M1.
Bronchial inflammation is known to be an important component in the pathogenesis of asthma. Inflammation occurs in both large and small airways and also causes an associated increase in airway responsiveness to a variety of inhaled stimuli. In clinical trials, ciclesonide has been shown to reduce airway reactivity to adenosine monophosphate in hyperreactive patients. Pre-treatment with ciclesonide for seven days significantly attenuated the early and late phase reactions following inhaled allergen challenge. Inhaled ciclesonide treatment was also shown to attenuate the increase in inflammatory cells (total eosinophils) and inflammatory mediators in induced sputum.
Pharmacodynamic Properties
Ciclesonide exhibits low binding affinity to the glucocorticoid-receptor. Once orally inhaled, ciclesonide is enzymatically converted in the lungs to the principal metabolite (C21-des-methylpropionyl-ciclesonide) which has a pronounced anti-inflammatory activity and is thus considered as the active metabolite. In four clinical trials, ciclesonide has been shown to reduce airway hyperresponsiveness to adenosine monophosphate in hyperreactive patients with maximal effect observed at the dose of 640 micrograms. In another trial, pretreatment with ciclesonide for seven days significantly attenuated the early and late phase reactions following inhaled allergen challenge. Inhaled ciclesonide treatment was also shown to attenuate the increase in inflammatory cells (total eosinophils) and inflammatory mediators in induced sputum.
A controlled study compared 24-hour plasma cortisol AUC in 26 adult asthmatic patients following 7 days of treatment. Compared to placebo, treatment with ciclesonide 320, 640, and 1,280 micrograms/day did not statistically significantly lower the 24-hour time averages of plasma cortisol (AUC(0-24)/24 hours) nor was a dose-dependent effect seen. In a clinical trial involving 164 adult male and female asthmatic patients, ciclesonide was given at doses of 320 micrograms or 640 micrograms/day over 12 weeks. After stimulation with 1 and 25 micrograms cosyntropin, no significant changes in plasma cortisol levels were observed versus placebo. Double-blind placebo-controlled trials of 12-weeks duration in adults and adolescents have shown that treatment with ciclesonide resulted in improved lung function as measured by FEV1 and PEF, improved asthma symptom control, and decreased need for inhaled beta-2 agonist.
In a 12-week study of 680 severe asthmatics, previously treated with 500-1,000 micrograms fluticasone propionate per day or equivalent, 87.3% and 93.3% of patients remained exacerbation-free during treatment with 160 or 640 micrograms of ciclesonide, respectively. At the end of the 12 week study period, the results showed a statistically significant difference between the doses of 160 micrograms and 640 micrograms/day ciclesonide with regard to the occurrence of an exacerbation after the first day of the study: 43 patients/339 (= 12.7%) in the 160 micrograms/day group and 23 patients/341 (6.7%) in the 640 micrograms/day group (Hazard ratio=0.526; p= 0.0134). Both ciclesonide doses resulted in comparable FEV1 values at 12 weeks. Treatment-related adverse events were seen in 3.8% and 5% of patients treated with 160 or 640 micrograms per day of ciclesonide respectively. A further 52 week trial involving 367 patients with mild to moderate asthma, was unable to demonstrate a significant difference in the effect of higher doses of Ciclesonide (320 or 640 mcg per day) as compared to a lower dose (160 mcg per day) on asthma control.
Pharmacokinetic Properties
Ciclesonide is formulated in HFA-134a propellant and ethanol as a solution aerosol, which demonstrates a linear relationship between different doses, puff strengths and systemic exposure.
Absorption:
Studies with oral and intravenous dosing of radiolabeled ciclesonide have shown an incomplete extent of oral absorption (24.5%). The oral bioavailability of both ciclesonide and the active metabolite is negligible (<0.5% for ciclesonide, <1%for the metabolite). Based on a γ-scintigraphy experiment, lung deposition in healthy subjects is 52%. The systemic bioavailability for the active metabolite is >50% by using the ciclesonide metered dose inhaler. As the oral bioavailability for the active metabolite is <1%, the swallowed portion of the inhaled ciclesonide does not contribute to systemic absorption.
Distribution:
Following intravenous administration to healthy subjects, the initial distribution phase for ciclesonide was rapid and consistent with its high lipophilicity. The volume of distribution averaged 2.9 l/kg. The total serum clearance of ciclesonide is high (average 2.0 l/h/kg) indicating a high hepatic extraction. The percentage of ciclesonide bound to human plasma proteins averaged 99%, and that of the active metabolite 98-99%, indicating an almost complete binding of circulating ciclesonide/active metabolite to plasma proteins.
Metabolism:
Ciclesonide is primarily hydrolysed to its biologically active metabolite by esterase enzymes in the lung. Investigation of the enzymology of further metabolism by human liver microsomes showed that this compound is mainly metabolized to hydroxylated inactive metabolites by CYP3A4 catalysis. Furthermore, reversible lipophilic fatty acid ester conjugates of the active metabolite were detected in the lung.
Excretion:
Ciclesonide is predominantly excreted via the faeces (67%), after oral and intravenous administration, indicating that excretion via the bile is the major route of elimination.
Pharmacokinetic characteristics in patients:
Asthmatic patients
Ciclesonide shows no pharmacokinetic changes in mild asthmatic patients compared to healthy subjects.
Elderly According to population pharmacokinetics, age has no impact on the systemic exposure of the active metabolite.
Renal or hepatic impairment
Reduced liver function may affect the elimination of corticosteroids. In a study including patients with hepatic impairment suffering from liver cirrhosis, a higher systemic exposure to the active metabolite was observed.
Due to the lack of renal excretion of the active metabolite, studies on renal impaired patients have not been performed.
Non-Clinical Properties
Carcinogenesis, mutagenesis and impairment of fertility
Ciclesonide demonstrated no carcinogenic potential in a study of oral doses up to 900 mcg/kg/day (approximately 6 times the maximum human daily inhalation dose based on mcg/m2/day) in mice for 104 weeks and in a study of inhalation doses up to 193 mcg/kg/day (approximately 2 times the maximum human daily inhalation dose based on mcg/m2/day) in rats for 104 weeks. Ciclesonide was not mutagenic in an Ames test or in a forward mutation assay and was not clastogenic in a human lymphocyte assay or in an in vitro micronucleus test. However, ciclesonide was clastogenic in the in vivo mouse micronucleus test. The concurrent reference corticosteroid (dexamethasone) in this study showed similar findings. No evidence of impairment of fertility was observed in a reproductive study conducted in male and female rats both dosed orally up to 900 mcg/kg/day (approximately 10 times the maximum human daily inhalation dose based on mcg/m2/day).
Description
The active component of CICLOHALE 80 mcg Inhalation Aerosol, and CICLOHALE 160 mcg Inhalation Aerosol is ciclesonide, a non-halogenated glucocorticoid having the chemical name pregna-1,4-diene-3,20-dione,16,17-[[(R)-cyclohexylmethylene]bis(oxy)]-11-hydroxy-21(2-methyl-1-oxopropoxy)-,(11beta,16alpha). The empirical formula is C32H44O7 and its molecular weight is 540.7. Its structural formula is as follows:
Ciclesonide is a white to yellow-white powder. It is soluble in dehydrated alcohol, acetone, dichloromethane, and chloroform. CICLOHALE 80 mcg Inhalation Aerosol and CICLOHALE160 mcg Inhalation Aerosol are pressurized, metered-dose aerosol units. CICLOHALE is intended for oral inhalation only. Each unit contains a solution of ciclesonide in propellant HFA-134a (1,1,1,2 tetrafluoroethane) and ethanol. After priming, CICLOHALE 80 mcg delivers 100 mcg from the valve and 80 mcg of ciclesonide from the actuator. CICLOHALE 160 mcg delivers 200 mcg from the valve and 160 mcg of ciclesonide from the actuator. The actual amount of drug delivered to the lung may depend on patient factors, such as the coordination between the actuation of the device and inspiration through the delivery system.
Pharmaceutical Properties
Incompatibilities
Not applicable
Shelf Life
As on the pack
Storage and Handling Instructions
Store below 30O C.
Do not freeze
Patient Counselling Information
CICLOHALE (Ciclesonide) Inhalation Aerosol
Note: For Oral Inhalation Only
Do not use your CICLOHALE Inhalation Aerosol near heat or an open flame.
Read this Patient Information leaflet before you start using CICLOHALE Inhalation Aerosol and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment. If you have any questions about CICLOHALE Inhalation Aerosol, ask your healthcare provider.
What is CICLOHALE Inhalation Aerosol?
CICLOHALE Inhalation Aerosol is a prescription medicine used for the control and prevention of asthma in adults and children 12 years of age and older.
CICLOHALE Inhalation Aerosol contains ciclesonide, which is a man-made (synthetic) corticosteroid. Corticosteroids are natural substances found in the body and reduce inflammation. When you inhale CICLOHALE Inhalation Aerosol it may help to control and prevent your symptoms of asthma by reducing your airway inflammation.
CICLOHALE Inhalation Aerosol is not for the relief of acute bronchospasm. CICLOHALE Inhalation Aerosol is not a bronchodilator and does not treat sudden symptoms of an asthma attack such as wheezing, cough, shortness of breath, and chest pain or tightness. Always have a fast-acting bronchodilator medicine (rescue inhaler) with you to treat sudden symptoms.
It is not known if CICLOHALE Inhalation Aerosol is safe and effective in children 5 years of age and younger.
Who should not use CICLOHALE Inhalation Aerosol?
Do not use CICLOHALE Inhalation Aerosol:
- to treat status asthmaticus or other sudden symptoms of asthma. CICLOHALE Inhalation Aerosol is not a rescue inhaler and should not be used to give you fast relief from your asthma attack. Always use a rescue inhaler such as salbutamol/levosalbutamol, during a sudden asthma attack.
- if you are allergic to ciclesonide or any of the ingredients in CICLOHALE Inhalation Aerosol.
What should I tell my healthcare provider before using CICLOHALE Inhalation Aerosol?
Before you use CICLOHALE Inhalation Aerosol tell your healthcare provider if you:
- have or have had eye problems such as increased ocular pressure, glaucoma, or cataracts.
- have any infections including tuberculosis or ocular herpes simplex.
- have not had or been vaccinated for chicken pox or measles.
- are pregnant or plan to become pregnant. It is not known if CICLOHALE Inhalation Aerosol will harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if CICLOHALE Inhalation Aerosol passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you are using CICLOHALE Inhalation Aerosol.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use CICLOHALE Inhalation Aerosol?
- Read the Instructions for Use provided in the cartonfor specific information about the right way to use CICLOHALE Inhalation Aerosol.
- Use CICLOHALE Inhalation Aerosol exactly as your healthcare provider tells you to use it. Do not take more of your medicine, or take it more often than your healthcare provider tells you.
- You must use CICLOHALE Inhalation Aerosol regularly. It may take 4 weeks or longer after you start using CICLOHALE Inhalation Aerosol for your asthma symptoms to get better. Do not stop using CICLOHALE Inhalation Aerosol even if you are feeling better, unless your healthcare provider tells you to.
- If your symptoms do not improve or get worse, call your healthcare provider.
- Your healthcare provider may prescribe a rescue inhaler for emergency relief of sudden asthma attacks. Call your healthcare provider if you have: an asthma attack that does not respond to your rescue inhaler or you need more of your rescue inhaler than usual.
- If you use another inhaled medicine, ask your healthcare provider for instructions on how to use it while you use CICLOHALE Inhalation Aerosol.
What are the possible side effects of CICLOHALE Inhalation Aerosol?
CICLOHALE Inhalation Aerosol may cause serious side effects, including: • Thrush (Candida), a fungal infection of your nose, mouth, or throat. Tell your healthcare provider if you have discomfort or pain in your throat, have hoarseness in your voice or have any redness or white colored patches in your mouth or throat. Rinse your mouth after you use your CICLOHALE Inhalation Aerosol.
- Immune system problems that may increase your risk of infections.
You are more likely to get infections if you take medicines that may weaken your body’s ability to fight infections. Avoid contact with people who have contagious diseases such as chicken pox or measles while you use CICLOHALE Inhalation Aerosol. Symptoms of an infection may include: fever, pain, aches, chills , feeling tired , nausea , vomiting
Adrenal insufficiency. Adrenal insufficiency is a condition in which the adrenal glands do not make enough steroid hormones. Your healthcare provider will follow you closely if you take steroids by mouth and are having them decreased (tapered) or you are being switched to CICLOHALE Inhalation Aerosol. People have died while steroids are being decreased and when people have been switched from steroids by mouth to inhaled steroids like CICLOHALE. If you are under stress, such as with surgery, after surgery or trauma, you may need steroids by mouth again.
Call your healthcare provider right away if you have the following symptoms of adrenal insufficiency: • tiredness • weakness • dizziness • nausea that does not go away • vomiting that does not go away
- Decreased bone mass (bone mineral density). People who use inhaled steroid medicines for a long time may have an increased risk of decreased bone mass which can affect bone strength. Talk to your healthcare provider about any concerns you may have about bone health.
- Slowed or delayed growth in children. A child’s growth should be checked regularly while using CICLOHALE Inhalation Aerosol.
- Eye problems such as glaucoma and cataracts. If you have a history of glaucoma or cataracts or have a family history of eye problems, you should have regular eye exams while you use CICLOHALE Inhalation Aerosol.
- Increased wheezing (bronchospasm) can happen right away after using CICLOHALE Inhalation Aerosol. Stop using CICLOHALE Inhalation Aerosol and use an inhaled fast-acting bronchodilator (rescue inhaler) right away.
Tell your healthcare provider right away so that a new medicine can be prescribed to control your asthma.
The most common side effects with CICLOHALE Inhalation Aerosol include:
- headache
- swelling of nose and throat (nasopharyngitis)
- swelling of the sinuses (sinusitis)
- throat pain
- upper respiratory infection
- joint pain (arthralgia)
- nasal congestion
- pain in arms, legs, and back
Tell your healthcare provider about any side effect that bothers you or that does not go away.
These are not all of the possible side effects with CICLOHALE Inhalation Aerosol. For more information, ask your healthcare provider. Call your doctor for medical advice about side effects.
If you experience any side-effects, talk to your doctor or pharmacist or write to drugsafety@cipla.com. You can also report side effects directly via the national pharmacovigilance program of India by calling on 1800 180 3024 or you can report to Cipla Ltd on 18002677779. By reporting side-effects, you can help provide more information on the safety of this product.
How should I store CICLOHALE Inhalation Aerosol?
Store your CICLOHALE Inhalation Aerosol at room temperature around 30OC. Do not freeze. Do not puncture the CICLOHALE Inhalation Aerosol canister. Do not store the CICLOHALE Inhalation Aerosol canister near heat or a flame. Do not throw the CICLOHALE Inhalation Aerosol canister into a fire or an incinerator. Keep CICLOHALE Inhalation Aerosol clean and dry at all times. Keep CICLOHALE Inhalation Aerosol and all medicines out of reach of children.
General Information about the safe and effective use of CICLOHALE Inhalation Aerosol
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use CICLOHALE Inhalation Aerosol for a condition for which it was not prescribed. Do not give your CICLOHALE Inhalation Aerosol to other people, even if they have the same symptoms that you have. It may harm them.
Details Of Manufacturer
Cipla Ltd.
Peninsula Business Park, Ganapatrao Kadam Marg,
Lower Parel West, Mumbai,
Maharashtra 400013
Details of Permission or License Number
721/456/MFG/DFDA/2019/1737
Date of Revision
10/11/2019
References
- Tari Haahtela, Olof Selroos, Paul M. O'Byrne. Revisiting early intervention in adult asthma. ERJ Open Res 2015; 1: 00022-2015.
- Claus F.Vogelmeier,ThomasHering, ThomasLewin. Efficacy and safety of ciclesonide in the treatment of 24,037 asthmatic patients in routine medical care. Respiratory Medicine (2011) 105, 186-194.
- Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: a systematic review and meta-analysis. Arch Intern Med 1999; 159:941–955
- 2 Wilson AM, Sims EJ, Lipworth BJ. Dose response with fluticasone propionate on adrenocortical activity and recovery of basal and stimulated responses after stopping treatment. Clin Endocrinol 1999; 50:329–335
- 4 Casale TB, Nelson HS, Stricker WE, et al. Suppression of hypothalamic-pituitary-adrenal axis activity with inhaled flunisolide and fluticasone propionate in adult asthma patients. Ann Allergy Asthma Immunol 2001; 87:379–385
- Fardon TC, Lee DKC, Haggart K, et al. Adrenal suppression with dry powder formulations of fluticasone propionate and mometasone furoate. Am J Respir Crit Care Med 2004; 170:960–966
- Todd GR, Acerini CL, Ross-Russell R, et al. Survey of adrenal crisis associated with inhaled corticosteroids in the United Kingdom. Arch Dis Child 2002; 87:457–461
- J Bourbeau, S J Bartlett. Patient adherence in COPD. Thorax. 2008 Sep; 63(9): 831–838.
- Carylne M. Averell, Richard. Stanford et al. Medication adherence in patients with asthma using once-daily versus twice-daily ICS/LABAs. Journal of asthma 2019; 1-11.
- Carlyne M. Averell , MS, SM,Richard H. Stanford Timothy J Schaffner, David P Skoner.Ciclesonide: a safe and effective inhaled corticosteroid for the treatment of asthma. J Asthma Allergy, 2009; 2: 25–32.
- H. Derendorf, R. Nave, A. Drollmann. Relevance of pharmacokinetics and pharmacodynamics of inhaled corticosteroids to asthma Eur Respir J 2006; 28: 1042–1050
- Colice G. L., Derendorf H., Shapiro G. Inhaled Corticosteroids: Is There an Ideal Therapy? http://www.medscape.com/viewprogram/2917_ pnt.
- Derendorf H. Corticosteroid Pharmacokinetic/ Pharmacodynamic parameters and their relationship to Safety and Efficacy. Allergy and Asthma Proc 26: 327-335, 2005.
- Allen D. B., Biclory L., Derendorf H. et al. The Ideal Inhaled Corticosteroid. J Allergy Clin Immunol 2003; 112: S1-S8.
- Product Monograph: Alvesco Canada; ciclesonide inhalation aerosol. Last accessed on 26th February2020.https://www.astrazeneca.ca/content/dam/azca/downloads/productinformation/alvesco-product-monograph-en.pdf.
- Kelly HW. Comparison of inhaled corticosteroids: an update. Ann Pharmacother. 2009 Mar;43(3):519-27.
- Omar S Usmani. Small Airways Dysfunction in Asthma: Evaluation and Management to Improve Asthma Control. Allergy Asthma Immunol Res. 2014 Sep; 6(5): 376–388.
- Sundeep S Salvi, Komalkirti K Apte, Raja Dhar et al. Asthma Insights and Management in India: Lessons Learnt from the Asia Pacific – Asthma Insights and Management (AP-AIM) Study. Journal of The Association of Physicians of India, 2015,63:36-43.
- Carr TF, Altisheh R, Zitt M. Small airways disease and severe asthma. World Allergy Organ J. 2017 Jun 21;10(1):20.
- Usmani OS, Singh D, Spinola M, Bizzi A, Barnes PJ. The prevalence of small airways disease in adult asthma: A systematic literature review. Respir Med. 2016 Jul; 116:19-27.
- Perez T, Chanez P, Dusser D, Devillier P. Small airway impairment in moderate to severe asthmatics without significant proximal airway obstruction. Respir Med. 2013 Nov;107(11):1667-74.
- Global Initiative For Asthma 2019. Last accessed on 26th February 2020. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf.
- Peter J. Barnes. Inhaled Corticosteroids. Pharmaceuticals (Basel). 2010 Mar; 3(3): 514–540.
- David Hodgson, John Anderson, Catherine Reynolds. A randomised controlled trial of small particle inhaled steroids in refractory eosinophilic asthma (SPIRA). Thorax. 2015 Jun; 70(6): 559–565.
- Lavorini F, Pedersen S et al. Dilemmas, Confusion, and Misconceptions Related to Small Airways Directed Therapy. Chest. 2017 Jun;151(6):1345-1355.
- Martin RJ. Therapeutic significance of distal airway inflammation in asthma. J Allergy Clin Immunol. 2002 Feb;109(2 Suppl):S447-60.
- Steve Newman, Andrew Salmona et al. High lung deposition of 99mTc-labeled ciclesonide administered via HFA-MDI to patients with asthma. Respiratory Medicine (2006) 100, 375–384.
- Virendra Singh et al. Poster-P1242 presented at ERS 2006-Munich, Germany.
- J. Cohen, W.R. Douma et al. Ciclesonide improves measures of small airway involvement in asthma. Eur Respir J 2008; 31: 1213–1220.
- Claus F. Vogelmeier, Thomas Hering et al. Efficacy and safety of ciclesonide in the treatment of 24,037 asthmatic patients in routine medical care. Respiratory Medicine (2011) 105, 186-194.
- Brian J O’Connor, Stephen Kilfeather et al. Efficacy and safety of ciclesonide in patients with severe asthma: a 12-week, double-blind, randomized, parallel-group study with long-term (1-year) follow-up. Expert Opin. Pharmacother. (2010) 11(17):2791-2803.
- Roland Buhl, Ilona Vinkler et al. Comparable efficacy of ciclesonide once daily versus fluticasone propionate twice daily in asthma. Pulmonary Pharmacology & Therapeutics 19 (2006) 404–412.
- Kuo-Chin Chiu, Yen-Li Chou et al. Comparison of the efficacy of ciclesonide with that of
budesonide in mild to moderate asthma patients after step-down therapy: a randomised parallel-group study. npj Primary Care Respiratory Medicine (2014) 24;1-7.
- Erwin w. Gelfand, md, John w. Georgitis. Once-daily ciclesonide in children: efficacy and safety in asthma. J Pediatr 2006;148:377-83.
- Soren Pedersen, Renate Engelsta. Efficacy and safety of ciclesonide once daily and fluticasone propionate twice daily in children with asthma. Pulmonary Pharmacology & Therapeutics 22 (2009) 214–220.
- J.H. Vermeulena, K. Gyurkovits. Randomized comparison of the efficacy and safety of ciclesonide and budesonide in adolescents with severe asthma. Respiratory Medicine (2007) 101, 2182–2191.
- ALVESCO Prescribing Information. Last accessed on 26th February 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021658s006lbl.pdf.
- Brian J. Lipworth, MD*; Michael A. Effect of ciclesonide and fluticasone on hypothalamic-pituitary-adrenal axis function in adults with mild-to-moderate persistent asthma. Ann Allergy Asthma Immunol. 2005;94:465–472.
- Effects of ciclesonide and fluticasone on cortisol secretion in patients with persistent asthma. E. Derom, R. Louis, C. Tiesler. Eur Respir J 2009; 33: 1277–1286.
- Leo T. Chylack Jr. Gary N. Gross et al. A Randomized, Controlled Trial to Investigate the Effect of Ciclesonide and Beclomethasone Dipropionate on Eye Lens Opacity. Journal of Asthma,2008, 45:893–902.
- L. Agertoft, S. Pedersen. Lower-leg growth rate and HPA-axis function in children with asthma during treatment with inhaled ciclesonide. J allergy clin immunol. 113, 2; S119.
- Liddell BS, Oberlin JM, Hsu DP.Inhaled corticosteroid related adrenal suppression detected by poor growth and reversed with ciclesonide. J Asthma. 2017 ;54(1):99-104.