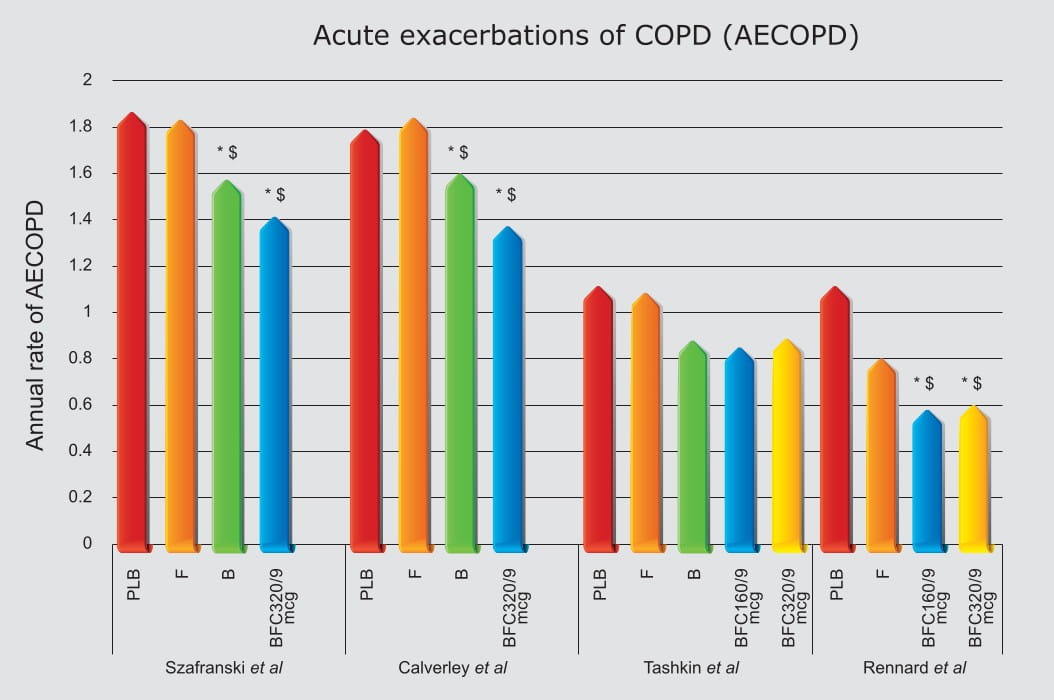
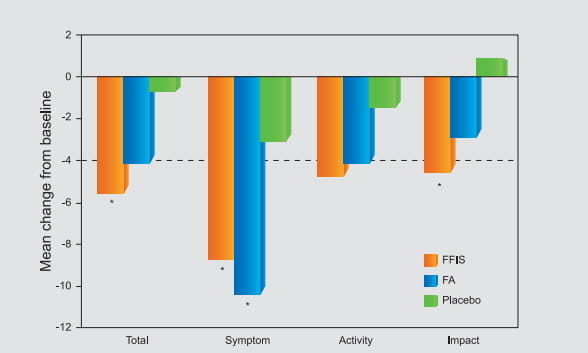
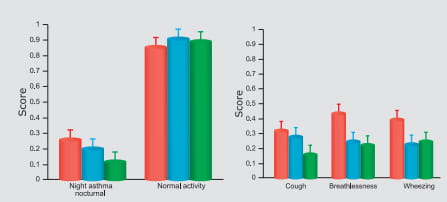
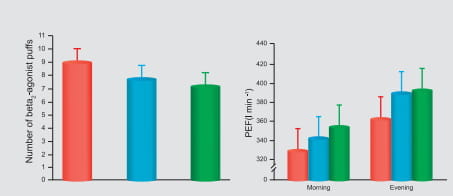
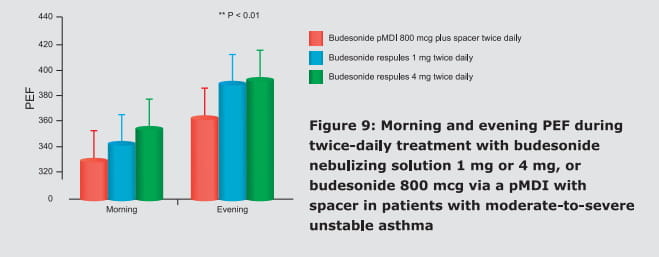
In fact, this combination is currently the only ICS/LABA combination available for inhalation in
not just a nebulized formulation but also a pMDI (including a breath-actuated inhaler) and dry
powder formulation, thus allowing a physician and the patient to decide as a team as to which
delivery system is the best for them.
For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only Formoterol
Fumarate Dihydrate and Budesonide Respules
FORACORT
FORACORT 0.5 mg Respules
Formoterol fumarate......20 mcg
Budesonide.................0.5 mg
FORACORT 1 mg Respules
Formoterol fumarate............20 mcg
Budesonide....................... 1 mg
Suspension for inhalation via a nebulizer
FORACORT Respules are a combination of budesonide, a potent glucocorticoid, and formoterol
fumarate, a selective, long-acting beta
2-adrenoceptor-agonist (beta
2 -agonist
[LABA]).
Budesonide is a potent glucocorticoid that binds with high affinity to the glucocorticoid
receptor. It has a high ratio of topical to systemic activity.
Formoterol fumarate is a very potent LABA with a high intrinsic activity and a rapid onset of
action.
Pharmacodynamics
FORACORT Respules contain an aqueous suspension of budesonide and formoterol fumarate,
both of which have different effects on the clinical, physiological and inflammatory indices of
asthma.
Budesonide
Budesonide is an anti-inflammatory corticosteroid that exhibits potent glucocorticoid activity
and weak mineralocorticoid activity. In standard in vitro and animal models, budesonide
has approximately a 200-fold higher affinity for the glucocorticoid receptor and a 1,000-fold
higher topical anti-inflammatory potency than cortisol.
In glucocorticoid-receptor affinity studies, the 22R form of budesonide was two times as active
as the 22S epimer. In vitro studies indicated that the two forms of budesonide do not
interconvert.
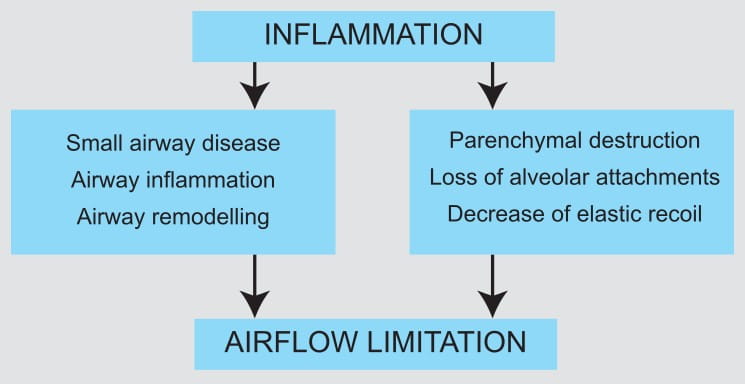
Inflammation is an important component in the pathogenesis of asthma. Corticosteroids have a wide
range of inhibitory activities against multiple cell types (e.g., mast cells, eosinophils,
neutrophils, macrophages and lymphocytes) and mediators (e.g., histamine, eicosanoids,
leukotrienes and cytokines) involved in allergic and non-allergic-mediated inflammation. These
anti-inflammatory actions of corticosteroids may contribute to their efficacy in asthma.
Studies in asthmatic patients have shown a favourable ratio between topical anti-inflammatory
activity and systemic corticosteroid effects over a wide range of doses of budesonide. This is
explained by a combination of a relatively high local anti-inflammatory effect, extensive
first-pass hepatic degradation of orally absorbed drug (85-95%), and the low potency of formed
metabolites.
Formoterol Fumarate
Formoterol fumarate is a selective LABA. Inhaled formoterol fumarate dihydrate acts locally in
the lungs as a bronchodilator. In vitro studies have shown that formoterol has more
than 200-fold greater agonist activity at beta2-receptors than at
beta1-receptors. Although beta2-receptors are the predominant adrenergic
receptors in bronchial smooth muscle and beta1-receptors are the predominant
receptors in the heart, there are also beta2-receptors in the human heart comprising
10-50% of the total beta-adrenergic-receptors. The precise function of these receptors has not
been established, but they raise the possibility that even highly selective
beta2-agonists may have cardiac effects.
The pharmacological effects of beta2-adrenoceptor-agonist drugs, including formoterol,
are at least in part attributable to the stimulation of intracellular adenyl cyclase, the enzyme
that catalyses the conversion of adenosine triphosphate (ATP) to cyclic-3', 5'-adenosine
monophosphate (cyclic AMP). Increased cyclic AMP levels cause relaxation of bronchial smooth
muscle and inhibit the release of mediators of immediate hypersensitivity from cells, especially
from mast cells.
In vitro tests show that formoterol is an inhibitor of the release of mast cell
mediators, such as histamine and leukotrienes, from the human lungs. Formoterol also inhibits
histamine-induced plasma albumin extravasation in anaesthetized guinea pigs and inhibits
allergen-induced eosinophil influx in dogs with airway hyper-responsiveness. The relevance of
these in vitro and animal findings to humans with chronic obstructive pulmonary disease
(COPD) is unknown.
Pharmacokinetics
Budesonide is primarily cleared by the liver. In asthmatic children, 4 to 6 years of age, the
terminal half-life of budesonide after nebulization is 2.3 hours and the systemic clearance is
0.5 L/min, which is approximately 50% greater than in healthy adults after adjustment for
differences in weight. Also, after a single dose of 1 mg budesonide, a peak plasma concentration
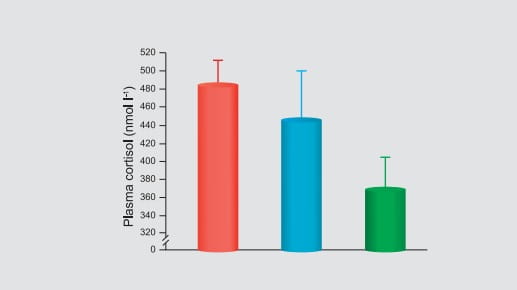
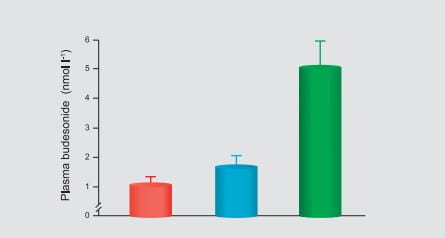
of 2.6 nmol/L was obtained approximately 20 minutes after nebulization. Moreover, the exposure
(AUC) of budesonide, following administration of a single 1 mg dose of budesonide by
nebulization, is comparable to healthy adults given a single 2 mg dose by nebulization.
Information on the pharmacokinetics of formoterol (dry powder and/or inhalation solution) in
plasma and/or urine is available in healthy subjects as well as patients with COPD after oral
inhalation of doses at and above the therapeutic dose. Urinary excretion of unchanged formoterol
was used as an indirect measure of systemic exposure. Plasma drug disposition data parallel
urinary excretion, and the elimination half-lives calculated for urine and plasma are similar.
Absorption
Budesonide
In asthmatic children, 4 to 6 years of age, the total absolute bioavailability (i.e., lungs plus
oral) following administration of budesonide respules via a jet nebulizer was approximately 6%
of the labelled dose. The peak plasma concentration of budesonide occurred 10-30 minutes after
the start of nebulization.
Formoterol Fumarate
Pharmacokinetic properties of formoterol fumarate were evaluated in 12 COPD patients following
inhalation of single doses of formoterol inhalation solution containing 10, 20 and 244 mcg of
formoterol sis) and 12 mcg formoterol fumarate dry powder, through 36 hours after single-dose
administration. Formoterol fumarate concentrations in plasma following the 10 mcg and 20 mcg
doses of formoterol inhalation solution and the 12 mcg dose of formoterol fumarate dry powder
were undetectable or only detected sporadically at very low concentrations. Following a single
244 mcg dose of formoterol inhalation solution (approximately 12 times the recommended clinical
dose), formoterol fumarate concentrations were readily measurable in plasma, exhibiting rapid
absorption into plasma, and reaching a maximum drug concentration of 72 pg/mL within
approximately 12 minutes of dosing. The mean amount of formoterol excreted unchanged in 24-hour
urine following single oral inhalation doses of 10, 20 and 244 mcg formoterol inhalation
solution were found to be 109.7 ng, 349.6 ng and 3317.5 ng, respectively. These findings
indicate a near dose-proportional increase in systemic exposure within the dose range tested.
When 12 mcg of a dry powder formulation of formoterol fumarate was given twice daily to COPD
patients by oral inhalation for 12 weeks, the accumulation index, based on the urinary excretion
of unchanged formoterol, was 1.19 to 1.38. This suggests some accumulation of formoterol in
plasma with multiple dosing. Although multiple-dose pharmacokinetic data is unavailable from
formoterol inhalation solution, assumption of linear pharmacokinetics allows a reasonable
prediction of minimal accumulation based on single-dose pharmacokinetics. As with many drug
products for oral inhalation, it is likely that the majority of the inhaled formoterol fumarate
delivered is swallowed and then absorbed from the gastrointestinal tract.
Distribution
Budesonide
In asthmatic children, 4 to 6 years of age, the volume of distribution of budesonide at the
steady state was 3 L/kg, approximately the same as in healthy adults. Budesonide is 85-90% bound
to plasma proteins, the degree of binding being constant over the concentration range (1 to 100
nmol/L) achieved with, and exceeding, recommended doses. Budesonide showed little or no binding
to corticosteroid-binding globulin. Budesonide rapidly equilibrated with red blood cells in a
concentration-independent manner, with a blood/plasma ratio of about 0.8.
Formoterol Fumarate
The binding of formoterol to human plasma proteins in vitro ranged from 61% to 64% at
concentrations from 0.1 to 100 ng/mL. Binding to human serum albumin in vitro was 31% to 38%
over a range of 5 to 500 ng/mL The concentrations of formoterol used to assess the plasma
protein binding were higher than those achieved in plasma following inhalation of a single 244
mcg dose of formoterol inhalation solution.
Metabolism
Budesonide
In vitro studies with human liver homogenates have shown that budesonide is rapidly and
extensively metabolized in the liver. Two major metabolites, formed via the cytochrome P450
(CYP450) isoenzyme 3A4 (CYP3A4)-catalysed biotransformation, have been isolated and identified
as 16-alpha-hydroxyprednisolone and 6-beta-hydroxybudesonide. The corticosteroid activity of
each of these two metabolites is less than 1% of that of the parent compound. No qualitative
difference between the In vitro and In vivo metabolic patterns has been detected. Negligible
metabolic inactivation was observed in the human lungs and in serum preparations.
Formoterol Fumarate
Formoterol fumarate is metabolized primarily by direct glucuronidation at either the phenolic
2'- or aliphatic-hydroxyl group, and O-demethylation followed by glucuronide conjugation at
either phenolic 2'-hydroxyl groups. Minor pathways involve sulphate conjugation of
formoterol and deformylation followed by sulphate conjugation. The most prominent pathway
involves direct conjugation at the phenolic 2'-hydroxyl group. The second major pathway
involves O-demethylation followed by conjugation at the phenolic 2'-hydroxyl group. In
vitro studies showed that multiple drug-metabolizing enzymes catalyse glucuronidation
(UGT1A1, 1A8, 1A9, 2B7 and 2B15 were the most predominant enzymes) and O-demethylation (CYP2D6,
CYP2C19, CYP2C9 and CYP2A6) of formoterol. Formoterol fumarate did not inhibit CYP450 enzymes at
therapeutically relevant concentrations. Some patients may be deficient in CYP2D6 or 2C19 or
both. It has not been adequately explored as to whether a deficiency in one or both of these
isozymes results in elevated systemic exposure to formoterol or systemic adverse effects.
Excretion
Budesonide
Budesonide is excreted in the urine and the faeces in the form of metabolites. In adults,
approximately 60% of an intravenous radiolabeled dose was recovered in the urine. No unchanged
budesonide was detected in the urine.
Formoterol Fumarate
Following administration, via a nebulizer, of single 10, 20 and 244 mcg formoterol inhalation
solution doses (calculated on an anhydrous basis) in 12 COPD patients, on average, about 1.1% to
1.7% of the dose was excreted in the urine as unchanged formoterol as compared to about 3.4%
excreted unchanged following inhalation administration of 12 mcg of formoterol fumarate dry
powder. Renal clearance of formoterol following inhalation administration of formoterol
inhalation solution in these subjects was about 157 mL/min. Based on plasma concentrations
measured following the 244 mcg dose, the mean terminal elimination half-life was determined to
be 7 hours.
Special Populations
Geriatric, Paediatric and Hepatic/Renal Impairment
The pharmacokinetics of FORACORT Respules has not been studied in elderly and paediatric
patient populations and in subjects with renal impairment. There are no data regarding the
specific use of the FORACORT Respules in patients with hepatic impairment. But since
formoterol and budesonide are primarily eliminated via hepatic metabolism, an increased exposure
can be expected in patients with severe liver impairment.
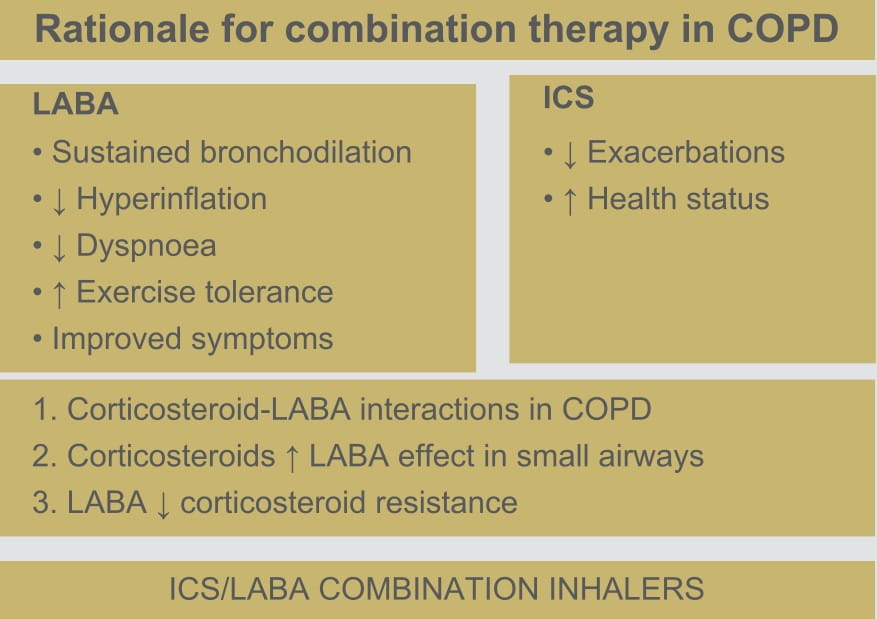
FORACORT Respules are indicated in the regular treatment of asthma, where the use of a
combination (LABA and inhaled corticosteroid [ICS]) has been found to be appropriate.
They are also indicated in the regular treatment of moderate-to-severe COPD, with frequent
symptoms and a history of repeated exacerbations despite regular therapy with long-acting
bronchodilators.
Asthma
Adults
FORACORT 0.5/1 mg Respules
One respule twice a day.
COPD
FORACORT 1 mg Respules
One respule twice a day.
Hypersensitivity to any ingredient of the formulation.
If patients find the treatment ineffective, or exceed the highest recommended dose of FORACORT
Respules, medical attention must be sought. Sudden and progressive deterioration in the
control of asthma is potentially life-threatening and the patient should undergo urgent medical
assessment. In this situation, consideration should be given to the need for increased therapy
with corticosteroids, e.g., a course of oral corticosteroids, or antibiotic treatment if an
infection is present. Patients should be advised to have their rescue inhaler available at all
times.
Patients should be reminded to take their FORACORT Respules maintenance dose as
prescribed, even when asymptomatic.
The prophylactic use of FORACORT Respules, e.g., before exercise, has not been studied.
For such use, a separate rapid-acting bronchodilator should be considered.
Once asthma symptoms are controlled, consideration may be given to gradually reducing the dose of
FORACORT Respules. It is recommended that the dose is tapered when the treatment is
discontinued and should not be stopped abruptly. Regular review of patients as treatment is
stepped down is important. The lowest effective dose of FORACORT Respules should be used.
Patients should not be initiated on FORACORT Respules during an exacerbation, or if they
have significantly worsening or acutely deteriorating asthma.
Serious asthma-related adverse events and exacerbations may occur during treatment with
FORACORT Respules. Patients should be asked to continue treatment but to seek medical
advice if asthma symptoms remain uncontrolled or worsen after initiation with FORACORT
Respules.
As with other inhalation therapy, paradoxical bronchospasm may occur, with an immediate increase
in wheezing after dosing. FORACORT Respules should then be discontinued; treatment should
be re-assessed and alternative therapy instituted if necessary. Systemic effects may occur with
any ICS, particularly at high doses prescribed for long periods. These effects are much less
likely to occur with inhalation treatment than with oral corticosteroids. Possible systemic
effects include adrenal suppression, growth retardation in children and adolescents, decrease in
bone mineral density, and cataract and glaucoma. It is recommended that the height of children
receiving prolonged treatment with ICS is regularly monitored. If growth is slowed, therapy
should be re-evaluated with the aim of reducing the dose of ICS. The benefits of the
corticosteroid therapy and the possible risks of growth suppression must be carefully weighed.
In addition consideration should be given to referring the patient to a paediatric respiratory
specialist.
Limited data from long-term studies suggest that most children and adolescents treated with
inhaled budesonide will ultimately achieve their adult target height. However, an initial small
but transient reduction in growth (approximately 1 cm) has been observed. This generally occurs
within the first year of treatment.
Long-term studies with inhaled budesonide in children at mean daily doses of 400 micrograms
(metered dose) or in adults at daily doses of 800 micrograms (metered dose) regarding the effect
of formoterol/budesonide at higher doses is available. If there is any reason to suppose that
adrenal function is impaired from previous systemic steroid therapy, care should be taken when
transferring patients to FORACORT Respules therapy.
The benefits of inhaled budesonide therapy would normally minimize the need for oral steroids,
but patients transferring from oral steroids may remain at risk of impaired adrenal reserve for
a considerable time. Patients who have required high-dose emergency corticosteroid therapy in
the past or prolonged treatment with high doses of ICS may also be at risk. Additional systemic
corticosteroid cover should be considered during periods of stress or elective surgery. To
minimize the risk of oropharyngeal Candida infection, the patient should be instructed to rinse
their mouth out with water after inhaling the maintenance dose. In some cases, facial skin
irritation has occurred when a nebulizer with a face mask has been used. To prevent such
irritation, the face should be washed after using the face mask.
FORACORT Respules should be administered with caution in patients with thyrotoxicosis,
phaeochromocytoma, diabetes mellitus, untreated hypokalemia, hypertrophic obstructive
cardiomyopathy, idiopathic subvalvular aortic stenosis, severe hypertension, aneurysm or other
severe cardiovascular disorders, such as ischaemic heart disease, tachyarrhythmias or severe
heart failure. Caution should be observed when treating patients with prolongation of the QTc
interval. Formoterol fumarate itself may induce prolongation of the QTc interval, and increase
pulse rate and systolic/diastolic blood pressure.
The need for, and the dose of, ICS should be re-evaluated in patients with active or quiescent
pulmonary tuberculosis, or fungal and viral infections in the airways.
Potentially serious hypokalaemia may result from high doses of beta2-agonists.
Concomitant treatment of beta2-agonists with drugs which can induce hypokalaemia or
potentiate a hypokalaemic effect, e.g., xanthine-derivatives, steroids and diuretics, may add to
a possible hypokalaemic effect of the beta2-agonist. Particular caution is
recommended in unstable asthma with variable use of rescue bronchodilators, in acute severe
asthma as the associated risk may be augmented by hypoxia, and in other conditions when the
likelihood for hypokalaemia adverse effects is increased. It is recommended that serum potassium
levels are monitored during these circumstances. As for all beta2-agonists,
additional blood glucose controls should be considered in diabetic patients.
Pharmacokinetic Interactions
The metabolic conversion of budesonide is impeded by substances metabolized by CYP450 3A4 (e.g.,
itraconazole, ketoconazole, ritonavir). The concomitant administration of these potent
inhibitors of CYP450 3A4 may increase plasma levels of budesonide. The concomitant use of these
drugs should be avoided unless the benefit outweighs the increased risk of systemic side
effects. At recommended doses, cimetidine had a slight but clinically insignificant effect on
the pharmacokinetics of oral budesonide.
In patients using potent CYP3A4 inhibitors, FORACORT Respules are not recommended.
Pharmacodynamic Interactions
Concomitant use of other beta-adrenergic drugs can have a potentially additive effect.
Hypokalaemia may increase the disposition towards arrhythmias in patients who are treated with
digitalis glycosides.
Beta-adrenergic blockers can weaken or inhibit the effect of formoterol. FORACORT Respules
should, therefore, not be given together with beta-adrenergic blockers (including eye drops)
unless there are compelling reasons. In such cases, cardioselective beta-blockers could be
considered, although they should be administered with caution.
Concomitant treatment with quinidine, disopyramide, procainamide, phenothiazines, antihistamines
(terfenadine), monoamine oxidase inhibitors and tricyclic antidepressants can prolong the QTc
interval and increase the risk of ventricular arrhythmias.
In addition, L-Dopa, L-thyroxine, oxytocin and alcohol can impair cardiac tolerance towards
beta2-sympathomimetics.
Caution is advised in the co-administration of beta-agonists with non-potassium-sparing diuretics
(such as loop or thiazide diuretics) as these may acutely worsen the electrocardiogram (ECG)
changes and/or hypokalaemia.
Concomitant treatment with xanthine derivatives or steroids may potentiate any hypokalaemic
effect.
There is an elevated risk of arrhythmias in patients receiving concomitant anaesthesia with
halogenated hydrocarbons.
Budesonide and formoterol have not been observed to interact with any other drugs used in the
treatment of asthma.
There are no adequate data from the use of formoterol and budesonide in pregnant women.
Administration of FORACORT Respules in pregnant women should only be considered if the
expected benefit to the mother is greater than any possible risk to the foetus. The lowest
effective dose of budesonide needed to maintain adequate asthma control should be used.
Budesonide is excreted in breast milk. However, at therapeutic doses, no effects on the
breastfeeding child are anticipated. It is not known whether formoterol passes into human breast
milk. Administration of FORACORT Respules to women who are breastfeeding should only be
considered if the expected benefit to the mother is greater than any possible risk to the child.
The growth of paediatric patients receiving ICS orally, including FORACORT Respules,
should be monitored. If a child or adolescent on any corticosteroid appears to have growth
suppression, the possibility that he/she is particularly sensitive to this effect should be
considered. The potential growth effects of prolonged treatment should be weighed against the
clinical benefits obtained. To minimize the systemic effects of ICS given orally, including
FORACORT Respules, each patient should be titrated to the lowest strength that
effectively controls his/her asthma.
No overall differences in safety were observed between these patients and younger patients. As
with other products containing beta2-agonists, special caution should be observed
when using FORACORT Respules in geriatric patients who have concomitant cardiovascular
disease that could be adversely affected by beta2-agonists.
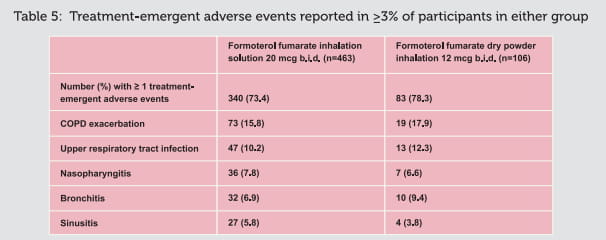
Since FORACORT Respules contains both budesonide and formoterol, the same pattern of
undesirable effects as reported for these substances may occur. No increased incidence of
adverse reactions has been seen following concurrent administration of the two compounds. The
most common drug-related adverse reactions are pharmacologically predictable side effects of
beta2-agonist therapy, such as tremor and palpitations. These tend to be mild and
usually disappear within a few days of treatment.
The common side effects observed in clinical trials with budesonide inhalation suspension and
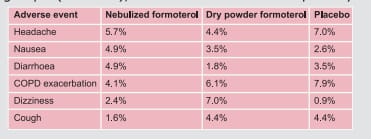
occurring at the incidence of ≥3% as compared to placebo were as follows: respiratory
infection, rhinitis, coughing, otitis media, viral infection, moniliasis, gastroenteritis,
vomiting, diarrhoea, abdominal pain, ear infection, epistaxis, and conjunctivitis and rash.
Following are some common, uncommon and rare adverse events that occurred in the groups
receiving formoterol/budesonide inhaler and formoterol and budesonide nebulizing
preparations:
Cardiac Disorders:
Palpitations, tachycardia, cardiac arrhythmias, e.g., atrial fibrillation, supraventricular
tachycardia, extrasystoles, angina pectoris.
Endocrine Disorders:
Signs or symptoms of systemic glucocorticosteroid effects, e.g., adrenal suppression, growth
retardation, decrease in bone mineral density, cataract and glaucoma, hypocorticism and
hypercorticism.
Gastrointestinal Disorders:
Nausea, diarrhoea, vomiting.
Immune System Disorders:
Immediate and delayed hypersensitivity reactions, e.g., exanthema, urticaria, pruritus,
dermatitis, angio-oedema and anaphylactic reactions, eye infection, herpes simplex, external ear
infection, infection, bronchospasm.
Infections and Infestations:
Candida infections in the oropharynx, sinusitis, pharyngitis, bronchitis.
Metabolic and Nutrition Disorders:
Hypokalemia, hyperglycaemia, anorexia.
Musculoskeletal, connective tissue and bone disorders:
Muscle cramps, myalgia, avascular necrosis of the femoral head, osteoporosis, growth
suppression.
Nervous System Disorders:
Headache, tremor, dizziness, taste disturbances, hyperkinesia.
Psychiatric Disorders:
Agitation, restlessness, nervousness, sleep disturbances, depression, behavioural disturbances
(mainly in children), psychosis, anxiety, irritability, aggressive reactions.
Respiratory, Thoracic and Mediastinal Disorders:
Mild irritation in the throat, dry mouth, coughing, hoarseness, bronchospasm, nasopharyngitis,
chest pain, dysphonia, stridor.
Skin and Subcutaneous Tissue Disorders:
Bruises, facial skin irritation, urticaria, rash, dermatitis, pruritus, purpura.
Vascular Disorders:
Variations in blood pressure.
Blood and Lymphatic System Disorders:
Cervical lymphadenopathy.
Ear and Labyrinth Disorders:
Earache.
General Disorders and Administration Site Conditions:
Fatigue, flu-like disorder, fever, pain.
Injury, Poisoning and Procedural Complication:
Fracture.
As with other inhalation therapy, paradoxical bronchospasm may occur in very rare cases.
Treatment with beta2-agonists may result in an increase in the blood levels of
insulin, free fatty acids, glycerol and ketone bodies.
An overdose of formoterol would likely lead to effects that are typical for
beta2-adrenergic agonists: Tremor, headache, palpitations, muscle cramps, dry mouth,
nausea, dizziness, fatigue, malaise, insomnia, tachycardia, hyperglycaemia, hypokalaemia,
prolonged QTc interval, angina, hypertension or hypotension, arrhythmia and vomiting. Supportive
and symptomatic treatment may be indicated. A dose of 90 micrograms administered during 3 hours
in patients with acute bronchial obstruction raised no safety concerns. Acute overdosage with
budesonide, even in excessive doses, is not expected to be a clinical problem. When used
continually in excessive doses, systemic glucocorticosteroid effects, such as hypercorticism and
adrenal suppression, may appear.
If FORACORT Respules therapy has to be withdrawn due to overdose of the formoterol
component of the drug, provision of appropriate ICS therapy must be considered.
See on the pack.
Store in the protective foil pouch under refrigeration at 2°-8°C.
FORACORT Respules 0.5/1 mg.......... Pack of 20 respules
FORACORT Respules are compatible with other COPD drugs in a nebulized form, such as
ambroxol (Inhalex Respules), N-acetylcysteine (Mucinac Respules) and ipratropium bromide
(Ipravent Respules) for co-administration.
1. Global Initiative for Obstructive Lung Diseases (GOLD) 2010 and
2011
2. Drugs 2004; 64 (4):431-441
3. Int J Chron Obstruct Pulmon Dis 2007; 2:107-116
4. Br J Pharmacol 2008; 153:1090-1104
5. Prim Care Resp J 2010; 19 (2):93-103.
6. Int J Chron Obstruct Pulmon Dis 2010; 5:357-366
7. Resp Med 2011; 105:930-938
8. Prim Care Resp J 2010; 19(1):10-20
9. Am J Med 2007 May; 120(5):435-41
10.Geriatr Nurs 2009 Jan-Feb; 30(1):45-9
11. Respir Med 2002; 96:375-381
12. US Respiratory Disease 2007 - October 2007, touch briefings.
13. Lung India 2010; 27 (4):230-235
14. Int J Chron Obstruct Pulmon Dis 2010; 5:223-232
15. Drugs 2000; 60 (5):1141-1178
16. Respir Med 2005; 99:836-849
17.COPD 2008; 5:97-104
18. Respir Med 1998; 92:44-49
19. Pulm Pharmacol Ther 2008; 21:818-823
20.COPD 2012; 9:58-72
21. Data on file 2012, Cipla Ltd.
22. Am J Health Syst Pharm 2011; 68:1221-32