Ginette 35 Monograph
Androgenic disorders are the most common endocrinopathy of women, affecting up to 10% of women of reproductive age. About 65% to 85% of hyperandrogenic women have polycystic ovarian syndrome (PCOS). Clinical manifestations of PCOS include hirsutism, acne, obesity, anovulation and menstrual irregularities.
Women with PCOS may have insulin resistance, excess adrenal and ovarian androgen production, and high LH levels. As researchers better understand the causes of PCOS, more specific treatments are being developed.
The standard therapeutic approach for treating PCOS focuses on treating the hyperandrogenism directly. Therapy aims to minimize symptoms and manage the condition to help reduce the risk of long-term health complications.
Diet modifications and exercise can help reduce symptoms. Other therapies include combination oral contraceptives (OCs), antiandrogens, insulin-sensitising agents, and cosmetic measures. OCs are effective treatment for hirsutism, acne, and menstrual irregularities and may help protect women with PCOS from the risk of endometrial cancer associated with chronic unopposed estrogen.1
Polycystic ovarian syndrome (PCOS) is the most common form of anovulatory infertility. Its association with menstrual disturbances and altered hormonal parameters leads many affected women to attend a gynaecology or infertility clinic.2,3
Diagnostic clinical features of PCOS include menstrual disturbances secondary to chronic anovulation or oligo-ovulation, and hirsutism or acne due to hyperandrogenaemia. Despite this classic concept, it is a heterogeneous disorder and exact diagnostic criteria remain contentious.
Table 1: PCOS: Diagnostic criteria

Evidence supports the hypothesis that decreased peripheral insulin sensitivity and consequent hyperinsulinaemia are pivotal in the pathogenesis of PCOS.3 The exact mechanism(s) of insulin resistance is uncertain, but a post-receptor defect in adipose tissue has been identified. Despite insulin resistance in adipose and skeletal muscle, the ovary remains relatively sensitive to insulin, and both insulin and insulin-like growth factor I have stimulatory effects on thecal androgen production.
Figure 1 shows how the relative excess of insulin or enhanced ovarian sensitivity to insulin, in combination with an elevated luteinising hormone concentration, brings about thecal hyperplasia, increased androgen secretion, arrest of follicular development, and therefore anovulation along with menstrual disturbance.
Figure 1: Probable mechanism whereby defects in insulin metabolism promote increased androgen activity at the level of the ovary1
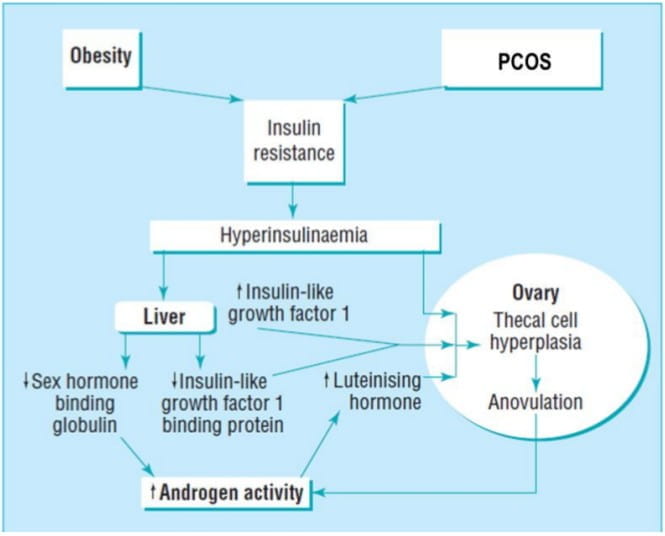
Insulin also acts on the liver to inhibit the production of sex hormone binding globulin (SHBG) and insulin-like growth factor I binding protein. A reduction in SHBG leads to an increase in the biologically free testosterone. Thus, insulin resistance not only increases secretion of ovarian androgens but also promotes an increase in the proportion of free (active) hormone. Similarly, inhibition of production of insulin-like growth factor I binding protein results in increased concentration of circulating free insulin-like growth factor I, further enhancing ovarian androgen production.
Current consensus suggests that the ovary is the principal site of excess androgen production, but some women with PCOS may have an andrenal contribution to the increased androgen production. The mechanisms remain obscure and are almost certainly multifactorial.2,3
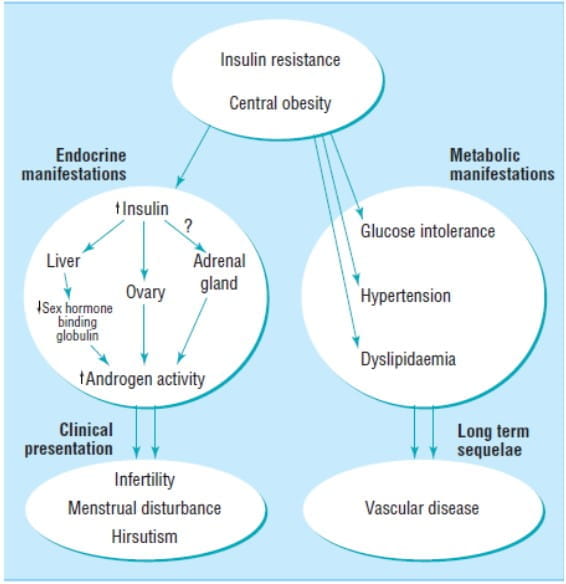
Figure 2 shows the principal features of PCOS.
Figure 2: Central role of insulin resistance: Clinical presenting features and long-term sequelae of PCOS2
Women with PCOS are currently treated according to their presenting features - irregular menses, hirsutism, or infertility (Table 2).
Table 2: PCOS - Current treatments
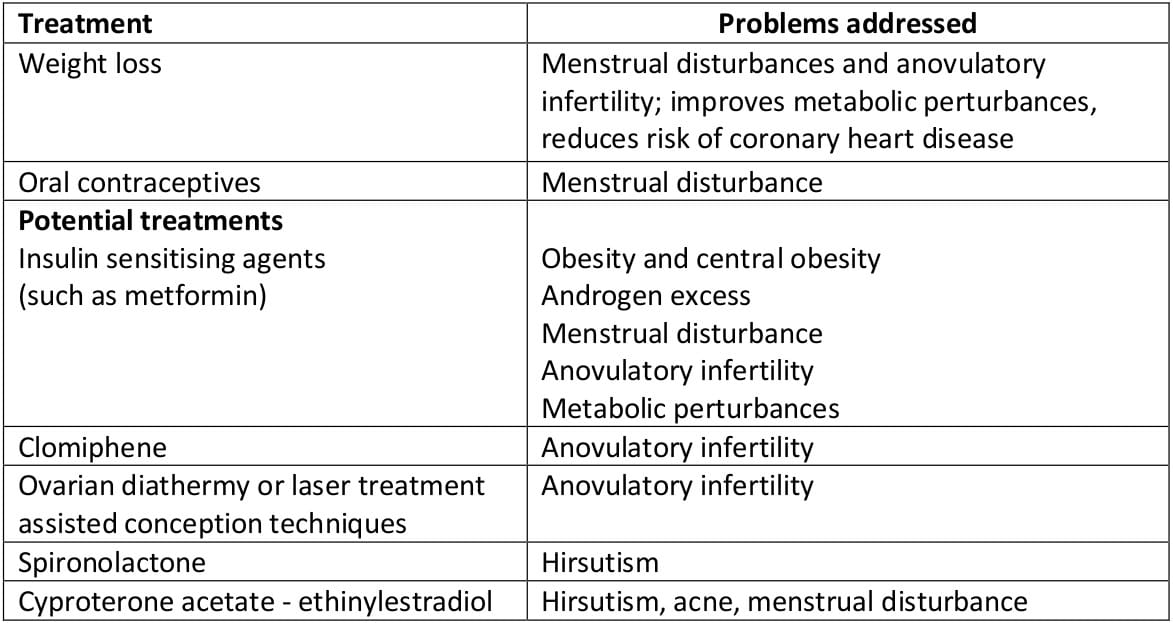
- Irregular menses - The combined oral contraceptive pill is commonly used to regulate menses. By increasing levels of SHBG while decreasing androgen secretion, it reduces the circulating free testosterone activity. [However, the combined pill exacerbates insulin resistance, and since many patients are overweight and obesity is a contraindication, this treatment may be unsuitable].
- Hirsutism - This problem may be addressed by the use of the antiandrogens cyproterone acetate or spironolactone (the former used in combination with ethinylestradiol). Their principal mode of action is the inhibition of the binding of dihydrotestosterone to its receptor at the hair follicle. Beneficial effects can be seen after three months, but excessive hair growth returns soon after cessation of treatment. Cyproterone acetate may exacerbate irregularity of the menstrual cycle.
- Infertility - For patients wishing to become pregnant, clomiphene citrate may stimulate ovulation but presents an increased risk of multiple pregnancy. By inhibiting the estrogen-mediated negative feedback loop at the hypothalamus, it enhances the secretion of follicle stimulating hormone (FSH). Guidelines suggest that the duration of clomiphene treatment should not exceed six months because of the potential increased risk of ovarian cancer. Those failing to conceive after clomiphene treatment usually respond to exogenous gonadotrophins, but this requires intensive monitoring to reduce the risk of multiple conceptions.
- Alternatives to medical treatment include laser or electrocautery of the ovary. This is often used as a last resort, is not available in all centres, and is difficult with obese patients. Although effective in aiding ovulation and regulating menses, its beneficial effects are usually short term.
- Insulin sensitising agents - Recent trials have investigated the effect of such agents on PCOS. Metformin, a biguanide often used in non-insulin dependent diabetes, has been the most commonly used agent.
Preliminary evidence indicates that treatment with metformin restores regular menstrual cycle and ovulation in obese women with PCOS.4
Ginette 35 (cyproterone acetate and ethinylestradiol) is a combination of antiandrogen-estrogen for use in the treatment of androgen-dependent conditions in women.
The development of an antiandrogen-containing formulation for the treatment of acne, seborrhea and hirsutism in women was based on the following principles:
- Androgens are known to cause the development of these skin conditions.
- Cyproterone acetate is a very strong antiandrogen, which competitively inhibits the binding of androgens to their receptors in the target organs.
- Ovarian production of androgens is reduced by the inhibition suppression of LH pulsatiles, which is a property of progestogen-estrogen combinations such as those used for contraception.
- The estrogen component of such a combination, ethinylestradiol, causes an increase in SHBG and, therefore, a reduction in free plasma testosterone.
The combinations of cyproterone acetate and ethinylestradiol chosen for therapy were expected to possess the antiovulatory and other principal properties of combined oral contraceptives, and since the two types could not be used in conjunction, it was essential to find how far the properties of oral contraceptives were shared by the formulations of cyproterone acetate.
Cyproterone acetate
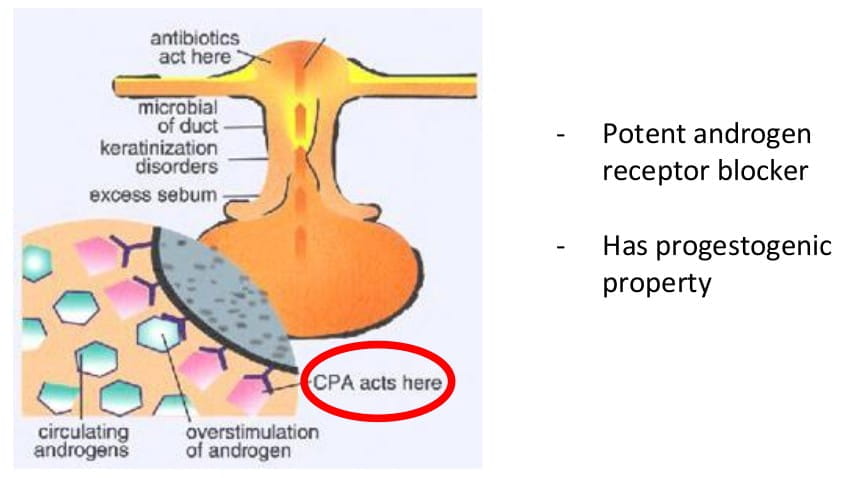
Cyproterone acetate is an analogue of hydroxyprogesterone and has progestational activity. It acts as a potent androgen receptor blocking agent and inhibits gonadotrophin secretion. Apart from this, cyrpoterone also has progestogenic activity.
Figure 3: mode of action of cyproterone
Ethinylestradiol
The estrogen component increases the level of SHBG, which in turn reduces the plasma level of free androgen. It also inhibits ovulation and therefore provides reliable contraception.
Following oral administration, cyproterone acetate is completely absorbed in a wide dose range. The ingestion of CPA-EE attains a maximum serum level of 15 ng cyproterone acetate/ml at 1.6 hours. Thereafter, drug serum levels decrease in two disposition phases characterized by half-lives of 0.8 hours and 2.3 days. The total clearance of cyproterone acetate from serum was determined to be 3.6 ml/min/kg. Cyproterone acetate is metabolized by various pathways, including hydroxylations and conjugations. The main metabolite in human plasma is the 15 beta-hydroxy derivative.
Some dose parts are excreted unchanged with the bile fluid. Most of the dose is excreted in the form of metabolites at a urinary to biliary ratio of 3:7. The renal and biliary excretion was determined to proceed with a half-life of 1.9 days. Metabolites from plasma were eliminated at a similar rate (half-life of 1.7 days). Cyproterone acetate is almost exclusively bound to plasma albumin. About 3.5-4.0% of total drug levels are present unbound. Because protein binding is non-specific, changes in the SHBG levels do not affect cyproterone acetate pharmacokinetics.
According to the long half-life of the terminal disposition phase from plasma (serum) and the daily intake, cyproterone acetate accumulates during one treatment cycle. Mean maximum drug serum levels increased from 15 ng/ml (day 1) to 21 ng/ml and 24 ng/ml at the end of the treatment cycles 1 and 3, respectively. The area under the concentration versus time profile increased 2.2-fold (end of cycle 1) and 2.4-fold (end of cycle 3). Steady- state conditions were reached after about 16 days. During long-term treatment, cyproterone acetate accumulates over treatment cycles by a factor of 2.
The absolute bioavailability of cyproterone acetate is almost complete (88% of the dose). The relative bioavailability of cyproterone acetate from CPA-EE was 109% when compared to an aqueous microcrystalline suspension.
Orally administered ethinylestradiol is rapidly and completely absorbed. Following ingestion of CPA-EE, maximum drug serum levels of about 80 pg/ml are reached at 1.7 hours. Thereafter, ethinylestradiol plasma levels decrease in two phases characterized by half-lives of 1-2 hours and about 20 hours. For analytical reasons, these parameters can only be calculated for higher dosages.
For ethinylestradiol, an apparent volume of distribution of about 5 l/kg and a metabolic clearance rate from plasma of about 5 ml/min/kg were determined.
Ethinylestradiol is highly but non-specifically bound to serum albumin. 2% of the drug levels are present unbound. During absorption and first passage through the liver, ethinylestradiol is metabolized resulting in a reduced absolute and variable oral bioavailability. Unchanged drug is not excreted. Ethinylestradiol metabolites are excreted at a urinary to biliary ratio of 4:6, with a half-life of about 1 day.
According to the half-life of the terminal disposition phase from plasma and the daily ingestion, steady-state plasma levels are reached after 3-4 days and are higher by 30-40% as compared to a single dose. The relative bioavailability (reference: aqueous microcrystalline suspension) of ethinylestradiol was almost complete.
The systemic bioavailability of ethinylestradiol might be influenced in both directions by other drugs. There is, however, no interaction with high doses of vitamin C.
Ethinylestradiol induces the hepatic synthesis of SHBG and corticosteroid binding globulin (CBG) during continuous use. The extent of SHBG induction, however, is dependent upon the chemical structure and dose of the co-administered progestin. During treatment with CPA-EE, SHBG concentrations in serum increased from about 100 nmol/l to 300 nmol/l and the serum concentrations of CBG were increased from about 50 μg/ml to 95 μg/ml.
Combination oral contraceptives have been used successfully for decades to address many of the immediate problems that women with hyperandrogenism face. The combination of cyproterone and ethinylestradiol helps to restore menstrual regularity, and treats hirsutism, acne, seborrhea and androgenic alopecia. The American College of Obstetricians and Gynaecologists acknowledges the benefits of OCs for treatment of hirsutism: 'The mainstay of medical therapy (for hirsutism) is low dose combination oral contraceptives that effectively suppresses ovarian function and reduces ovarian androgen secretion.'
A multicentre observational study was conducted to establish the therapeutic efficacy, contraceptive reliability and tolerance of antiandrogen-estrogen combination.
In all 1,161 women participated in the study. Of these, 613 were in the age group of 20 to 30 years. 238 women were below the age of 20 years. 294 were aged 30 to 50 and 4 were over 50 years of age.
The combination had a rapid and favourable effect on acne and seborrhea.
Table 3: Healing/improvement of all acne cases
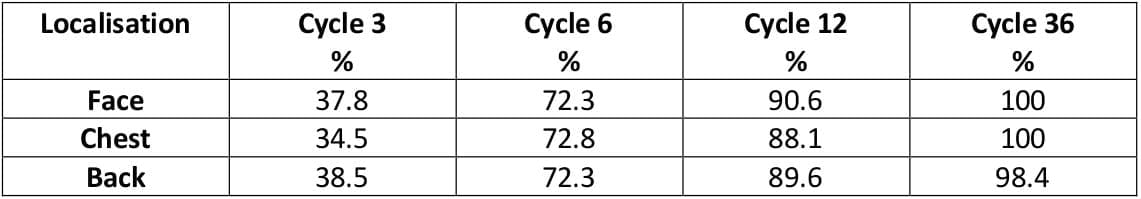
Figure 4: Time for normalization of greasy skin
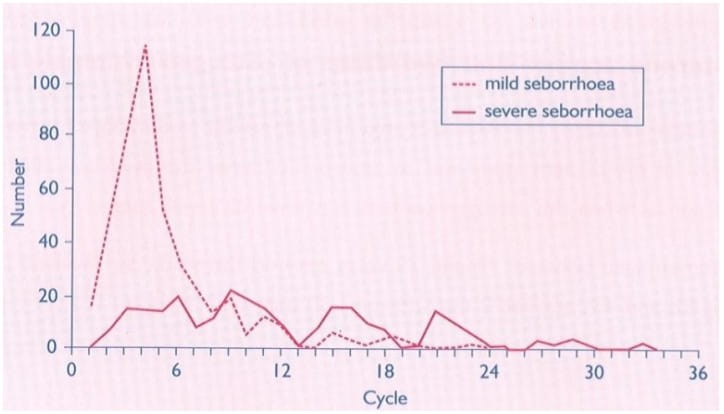
Severe dysmenorrhoea was almost completely abolished.
Table 4: Dysmenorrhoea at various stages

This study confirmed that the combination had rapid therapeutic effect. There was no evidence of tachyphylaxis. The therapeutic effect was sustained during long-term use indicated in some cases. Only two pregnancies occurred, both of which were definitely due to patient error. The cycle control and tolerance of the preparation were good and inter-menstrual bleeding was very rare.
Cyproterone acetate (CPA) has been available in combination of 50 mcg of ethinylestradiol (EE). The aim of the present study was to ascertain whether the beneficial effect of 2 mg CPA would persist when the estrogen content was lowered to 35 mcg.
In all, 96 women aged between 16 and 35 years entered the study, 50 received 2 mg CPA plus 35 mcg EE and 46 received 2 mg CPA plus 50 mcg EE.
Table 5: Visual analogue scale scores of acne severity before and after treatment Values are mean ± SD
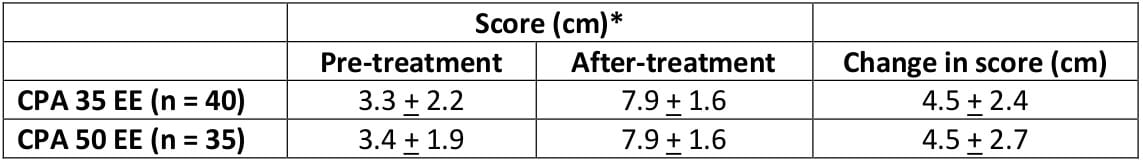
The changes from baseline in the number of patients suffering from acne were statistically significant in both treatment groups at all assessments after the third cycle. The preparation containing the lower dose of ethinylestradiol (35 mcg) appeared to offer advantages since efficacy remained unaltered while side effects, mostly estrogenic in origin, were reduced.
48 hyperandrogenic women were prospectively randomized to the following treatment for one year:
- CPA 2 mg with 35 mcg EE
- CPA 50 mg with 25 mcg EE
- Flutamide 250 mg daily
- Finasteride 5 mg daily
Acne was assessed by a modified Cook method (score from 0 to 9 for diffusion of the lesions on the face and from 0 to 9 for severity of the lesion).
Figure 5: Percentage reduction in Cook scores after 1 year of treatment with low dose
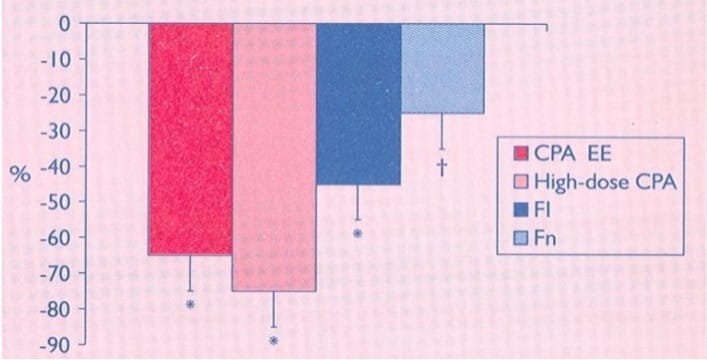
Low and high doses of CPA with EE was equally effective and was comparable to the effects of a low dose of flutamide (FI). Finasteride (Fn) was less beneficial.
An open, randomised, multicentre study was carried out to compare 2 mg CPA with 0.035 mg EE, and 0.150 mg desogestrel (DG) with 0.03 mg EE.
The duration of therapy was nine months. The combination of CPA-EE was used by 83 patients for 658 cycles and the combination of DG-EE by 79 women for 618 cycles.
Table 6: Healing/improvement of all cases of acne
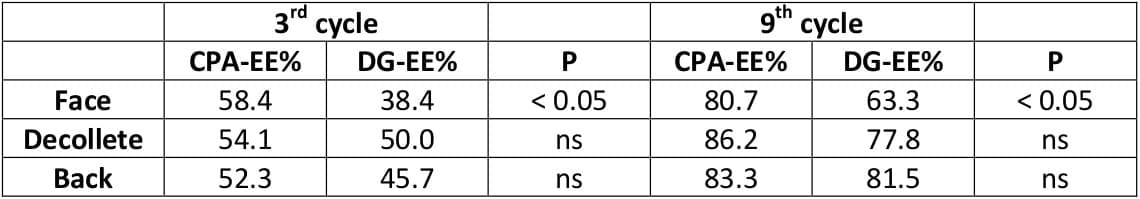
Figure 6: Normalization of seborrhea
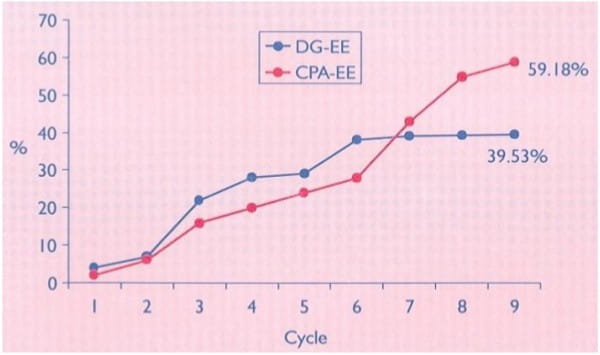
No pregnancy occurred under either therapy. Both preparations were well tolerated. However, some side effects, such as reduced libido, nervousness and breast tenderness were observed more frequently (p < 0.05) in the DG-EE group than among the users of the CPA-EE combination. The therapeutic outcome was better in the CPA-EE group especially in cases of facial acnce (p < 0.05). Symptoms of seborrhea responded better to the CPA-EE therapy.
The results show that the CPA-EE combination is superior to the DG-EE combination in the treatment of acne and seborrhea.
A multicentre controlled trial involving 78 patients was undertaken to compare CPA-EE combination with minocycline (50 mg b.d.). Duration of treatment period was 24 weeks, with assessment at weeks 0, 8, 16 and 24. At each assessment, the numbers of comedones, papules and pustules on the face, neck, shoulders and back were recorded.
Figure 7: Median spot counts (A-comedones, B-papules, C-pustules) on face, neck, back and shoulders as determined after 0, 8, 16, 24 weeks of therapy
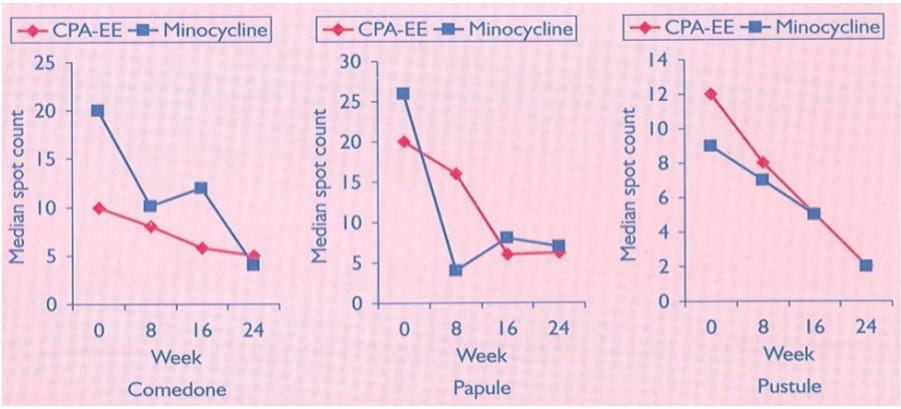
Table 7: Median lesion counts at 0 and 24 weeks (face, neck, back and shoulders)

Over a 24-week treatment period, CPA-EE and minocycline were found to be equally effective in reducing the lesion count for comedones, papules and pustules, and in the patient-subjective assessment of their skin. There was no significant change in weight or blood pressure in either treatment group and the number of patients who reported side effects was similar.
OCs are usually the first choice of treatment for PCOS, when fertility is not desired. However, they do not improve, or may even further induce impairment of insulin sensitivity, which is already impaired in women with PCOS. In this prospective, randomised study, we analysed the additional benefits of adding metformin to the OC treatment in non-obese women with PCOS.
Methods
After a baseline work-up including body mass indeed (BMI), waist : hip ratio (WHR), Ferriman - Gallwey score, ovarian volume, serum gonadotrophin, androgen and SHBG levels; and fasting lipid, glucose and insulin levels, 40 non-obese women with PCOS were assigned either to the OC or to the OC + metformin treatment by computer-assisted randomization. At the end of the four-month follow-up period, subjects were re-evaluated.
Figure 8: Baseline and after-treatment BMI values in the two groups*
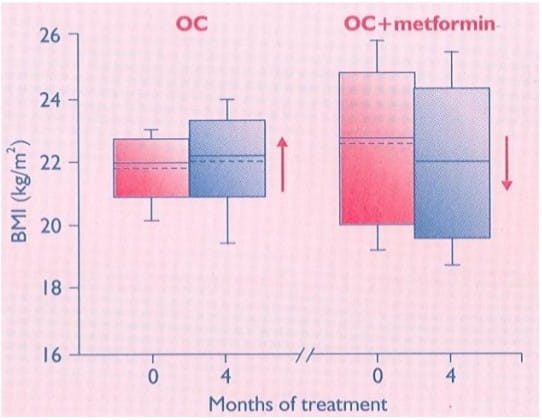
Figure 9: Baseline and after-treatment WHR in the two groups*
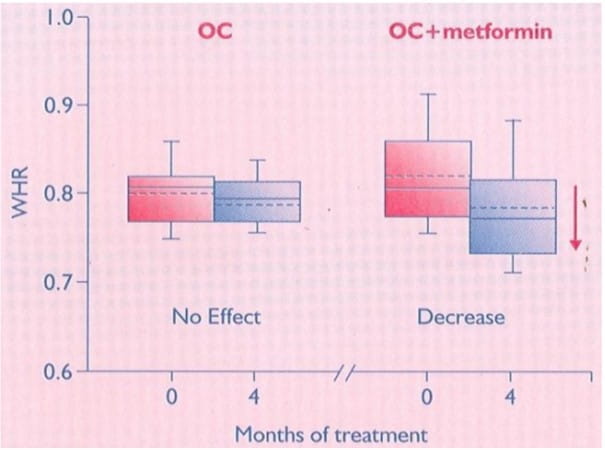
Figure 10: Baseline and after-treatment fasting glucose : insulin ratios in the two groups*
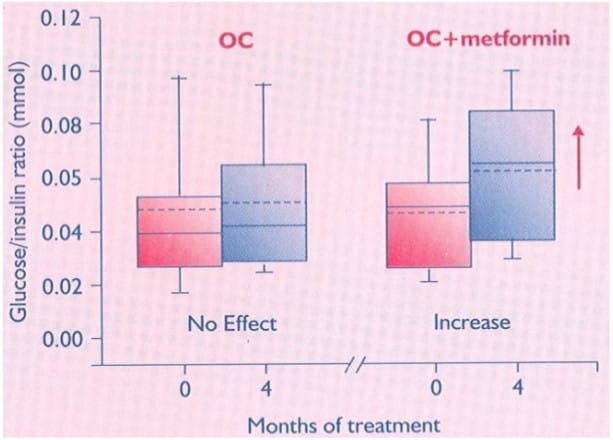
After treatment, women in the OC + metformin group had significant decreases in BMI and WHR, and a significant increase in insulin sensitivity in contrast to those in the OC group. Adding metformin to the OC treatment may improve the insulin sensitivity.
More than 10 years of clinical experience with CPA-EE has demonstrated the good tolerance and cycle control of this preparation. Estrogen-related adverse reactions occur less frequently with the CPA-EE combination.
In a three-year trial involving 1,161 women, yielding a total of 21,196 cycles, it was found that the CPA-EE preparation had a regulatory effect on the amount of flow and cycle length in women with a history of cycle irregularities.
The overall incidence of inter-menstrual bleeding was very low.
Table 8: Inter-menstrual bleeding (20,523 cycles)7
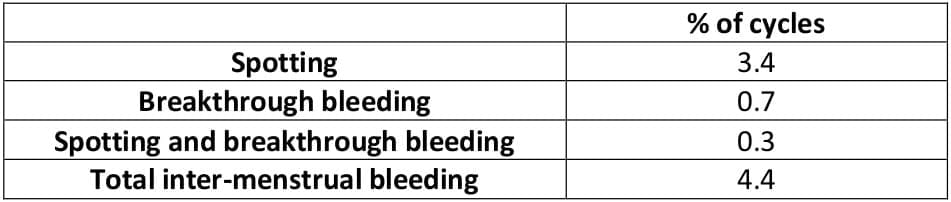
These results show that the cycle is extremely stable under CPA-EE. The preparation is marked by a particularly low rate of breakthrough bleeding.
In 26 women, the anticipated withdrawal bleeding failed to occur in a total of 59 cycles, giving an amenorrhoea rate of 0.3% of cycles, which is very low.
Severe dysmenorrhoea was almost completely abolished, and mild dysmenorrhoea greatly reduced, as with orthodox combined oral contraceptives.
Body weight determined after certain periods of time were compared with initial values. Fluctuations up to ± 2 kg were regarded as within the physiological range of variation. By this standard, body weight was unaffected by CPA-EE in 90.1% of women after the 3rd cycle, in 86.8% after the 6th, in 81.0% after the 12th, in 76.2% after the 24th and in 81.8% after the 36th cycle.
During the three-year study, 10 women were prematurely withdrawn from therapy because of hypertension.
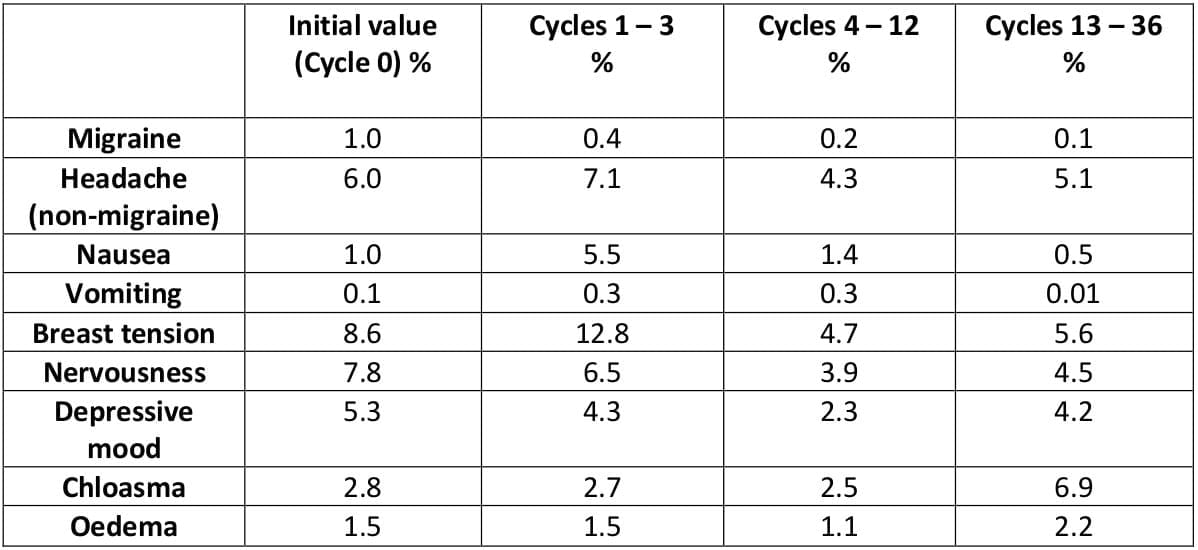
With the exception of nausea and breast tension in the short-term, none of the symptoms often ascribed to combined oral contraceptives appeared to increase. The incidence of chloasma increased slightly with long-term treatment.
Thrombophlebitis occurred in one woman, and possibly in another (0.01% of the total population). In the first, definite thrombophlebitis led to termination of therapy in the 3rd cycle. In the second, the symptoms were so mild that they were not regarded as a reason for termination. No severe thromboembolic complications were observed.
Symptoms believed to be attributable to disturbances of liver function were to be classified as 'hepatopathy'. Biliary complaints and, occasionally, severe nausea and/or vomiting, were the main reactions recorded in this class. Upper abdominal complaints were recorded as hepatopathy in two patients. However, they were mild, and did not lead to discontinuation of the medication.
Biochemical and ultrasonographic findings before the start and after the end of the study in a subgroup of 134 women indicated very good liver tolerance with long-term use.
Despite the length of the study, the drop-out rate for adverse reactions was only 11.7% (136 women).
Table 9: Concurrent symptoms and signs: Percentages of affected woman-cycles7
GINETTE 35 is indicated for the following:
- Androgen-dependent acne, especially those forms that are accompanied by seborrhoea or by inflammation or formation of nodes (acne papulopustulosa, acne nodulocystica)
- Androgen-dependent alopecia
- Mild forms of hirsutism
- Oral contraception in women requiring anti-androgen therapy
Although GINETTE 35 also acts as an oral contraceptive, it should not be used in women solely for contraception, but should be reserved for those women requiring treatment for the androgen-dependent conditions described.
Complete remission of acne is to be expected in nearly all cases, often within a few months, but in particularly severe cases, treatment for a longer duration may be necessary before the full benefit is seen. It is recommended that the treatment be withdrawn three to four cycles after the indicated condition(s) has/have completely resolved and that GINETTE 35 is not continued solely to provide oral contraception. Repeat courses of GINETTE 35 may be given if the androgen-dependent condition(s) recur.
GINETTE 35 inhibits ovulation and, thereby, prevents conception. Patients who are using GINETTE 35 should not, therefore, use an additional hormonal contraceptive, as this will expose the patient to an excessive dose of hormones and is not necessary for effective contraception.
First treatment course: One tablet daily for 21 days following the arrows, starting on the first day of the menstrual cycle (the first day of menstruation counting as day 1).
Subsequent courses: Each subsequent course is started after 7 tablet-free days have followed the preceding course.
When the contraceptive action of GINETTE 35 is also to be employed, it is essential that the above instructions be rigidly adhered to. Should bleeding fail to occur during the tablet-free interval, the possibility of pregnancy must be excluded before the next pack is started.
When changing from an oral contraceptive and relying on the contraceptive action of GINETTE 35, follow the instructions given below:
Changing from a 21-day pill pack: The first tablet of GINETTE 35 should be taken on the same day that a new pack of the previous pill pack would have been started.
Changing from a 28-day pill pack: The first tablet of GINETTE 35 should be taken on the same day that a new pack of the previous pill pack would have been started. Additional contraceptive precautions are not then required.
Changing from a progestogen-only pill (POP): The first tablet of GINETTE 35 should be taken on the first day of bleeding, even if a POP has already been taken on that day. Additional contraceptive precautions are not then required. The remaining POPs should be discarded.
Postpartum and post-abortum use: After pregnancy, GINETTE 35 can be started 21 days after a vaginal delivery, provided that the patient is fully ambulant and there are no puerperal complications. Additional contraceptive precautions will be required for the first 7 days of pill taking. Since the first postpartum ovulation may precede the first bleeding, another method of contraception should be used in the interval between childbirth and the first course of tablets. Lactation is contraindicated with GINETTE 35. After a first trimester abortion, GINETTE 35 may be started immediately, in which case no additional contraceptive precautions are required.
Special Circumstances Requiring Additional Contraception
Incorrect administration: A single delayed tablet should be taken as soon as possible, and if this can be done within 12 hours of the correct time, contraceptive protection is maintained. With longer delays, additional contraception is needed. Only the most recently delayed tablet should be taken, earlier missed tablets being omitted, and additional non- hormonal methods of contraception (except the rhythm or temperature methods) should be used for the next 7 days, while the next 7 tablets are being taken. Additionally, therefore, if tablet(s) have been missed during the last 7 days of a pack, there should be no break before the next pack is started. In this situation, a withdrawal bleed should not be expected until the end of the second pack. Some breakthrough bleeding may occur on the days a tablet is taken, but this is not clinically significant. If the patient does not have a withdrawal bleed during the tablet-free interval following the end of the second pack, the possibility of pregnancy must be ruled out before starting the next pack.
Gastrointestinal upset: Vomiting or diarrhoea may reduce the efficacy of oral contraceptives by preventing full absorption, but even so, tablets from the current pack should be continued. Additional non-hormonal methods of contraception (except the rhythm or temperature methods) should be used during the gastrointestinal upset and for 7 days following the upset. If these 7 days overrun the end of a pack, the next pack should be started without a break. In this situation, a withdrawal bleed should not be expected until the end of the second pack. If the patient does not have a withdrawal bleed during the tablet-free interval following the end of the second pack, the possibility of pregnancy must be ruled out before starting the next pack. Other methods of contraception should be considered if the gastrointestinal disorder is likely to be prolonged.
Each film-coated tablet contains:
Cyproterone acetate ............... 2 mg
Ethinylestradiol .......... 0.035 mg
Pharmacodynamics
GINETTE 35(cyproterone acetate and ethinylestradiol [CPA-EE]) blocks androgen receptors. It also reduces androgen synthesis both by a negative feedback effect on the hypothalamo- pituitary-ovarian systems and by the inhibition of androgen-synthesizing enzymes. Although GINETTE 35 also acts as an oral contraceptive, it is not recommended in women solely for contraception, but should be reserved for those women requiring treatment for the androgen-dependent skin conditions described.
Pharmacokinetics
Cyproterone acetate
Following oral administration, cyproterone acetate is completely absorbed in a wide dose range. The ingestion of CPA-EE attains a maximum serum level of 15 ng cyproterone acetate/ml at 1.6 hours. Thereafter, drug serum levels decrease in two disposition phases characterized by half-lives of 0.8 hours and 2.3 days. The total clearance of cyproterone acetate from serum was determined to be 3.6 ml/min/kg. Cyproterone acetate is metabolized by various pathways, including hydroxylations and conjugations. The main metabolite in human plasma is the 15 beta-hydroxy derivative.
Some dose parts are excreted unchanged with the bile fluid. Most of the dose is excreted in the form of metabolites at a urinary to biliary ratio of 3:7. The renal and biliary excretion was determined to proceed with a half-life of 1.9 days. Metabolites from plasma were eliminated at a similar rate (half-life of 1.7 days). Cyproterone acetate is almost exclusively bound to plasma albumin. About 3.5-4.0% of total drug levels are present unbound. Because protein binding is non-specific, changes in the sex hormone binding globulin (SHBG) levels do not affect cyproterone acetate pharmacokinetics.
According to the long half-life of the terminal disposition phase from plasma (serum) and the daily intake, cyproterone acetate accumulates during one treatment cycle. Mean maximum drug serum levels increased from 15 ng/ml (day 1) to 21 ng/ml and 24 ng/ml at the end of the treatment cycles 1 and 3, respectively. The area under the concentration versus time profile increased 2.2-fold (end of cycle 1) and 2.4-fold (end of cycle 3). Steady-state conditions were reached after about 16 days. During long-term treatment, cyproterone acetate accumulates over treatment cycles by a factor of 2.
The absolute bioavailability of cyproterone acetate is almost complete (88% of the dose). The relative bioavailability of cyproterone acetate from CPA-EE was 109% when compared to an aqueous microcrystalline suspension
Ethinylestradiol
Orally administered ethinylestradiol is rapidly and completely absorbed. Following ingestion of CPA-EE, maximum drug serum levels of about 80 pg/ml are reached at 1.7 hours. Thereafter, ethinylestradiol plasma levels decrease in two phases characterized by half-lives of 1-2 hours and about 20 hours. For analytical reasons, these parameters can only be calculated for higher dosages.
For ethinylestradiol, an apparent volume of distribution of about 5 l/kg and a metabolic clearance rate from plasma of about 5 ml/min/kg were determined.
Ethinylestradiol is highly but non-specifically bound to serum albumin. 2% of the drug levels are present unbound. During absorption and first passage through the liver, ethinylestradiol is metabolized resulting in a reduced absolute and variable oral bioavailability. Unchanged drug is not excreted. Ethinylestradiol metabolites are excreted at a urinary to biliary ratio of 4:6, with a half-life of about 1 day.
According to the half-life of the terminal disposition phase from plasma and the daily ingestion, steady-state plasma levels are reached after 3-4 days and are higher by 30-40% as compared to a single dose. The relative bioavailability (reference: aqueous microcrystalline suspension) of ethinylestradiol was almost complete.
The systemic bioavailability of ethinylestradiol might be influenced in both directions by other drugs. There is, however, no interaction with high doses of vitamin C.
Ethinylestradiol induces the hepatic synthesis of SHBG and corticosteroid binding globulin (CBG) during continuous use. The extent of SHBG induction, however, is dependent upon the chemical structure and dose of the co-administered progestin. During treatment with CPA-EE, SHBG concentrations in serum increased from about 100 nmol/l to 300 nmol/l and the serum concentrations of CBG were increased from about 50 μg/ml to 95 μg/ml.
GINETTE 35 is indicated for the following:
- Androgen-dependent acne, especially those forms that are accompanied by seborrhoea or by inflammation or formation of nodes (acne papulopustulosa, acne nodulocystica)
- Androgen-dependent alopecia
- Mild forms of hirsutism
- Oral contraception in women requiring anti-androgen therapy
Although GINETTE 35 also acts as an oral contraceptive, it should not be used in women solely for contraception, but should be reserved for those women requiring treatment for the androgen-dependent conditions described.
Complete remission of acne is to be expected in nearly all cases, often within a few months, but in particularly severe cases, treatment for a longer duration may be necessary before the full benefit is seen. It is recommended that the treatment be withdrawn three to four cycles after the indicated condition(s) has/have completely resolved and that GINETTE 35 is not continued solely to provide oral contraception. Repeat courses of GINETTE 35 may be given if the androgen-dependent condition(s) recur.
GINETTE 35 inhibits ovulation and, thereby, prevents conception. Patients who are using GINETTE 35 should not, therefore, use an additional hormonal contraceptive, as this will expose the patient to an excessive dose of hormones and is not necessary for effective contraception.
First treatment course: One tablet daily for 21 days following the arrows, starting on the first day of the menstrual cycle (the first day of menstruation counting as day 1).
Subsequent courses: Each subsequent course is started after 7 tablet-free days have followed the preceding course.
When the contraceptive action of GINETTE 35 is also to be employed, it is essential that the above instructions be rigidly adhered to. Should bleeding fail to occur during the tablet-free interval, the possibility of pregnancy must be excluded before the next pack is started.
When changing from an oral contraceptive and relying on the contraceptive action of GINETTE 35, follow the instructions given below:
Changing from a 21-day pill pack: The first tablet of GINETTE 35 should be taken on the same day that a new pack of the previous pill pack would have been started.
Changing from a 28-day pill pack: The first tablet of GINETTE 35 should be taken on the same day that a new pack of the previous pill pack would have been started. Additional contraceptive precautions are not then required.
Changing from a progestogen-only pill (POP): The first tablet of GINETTE 35 should be taken on the first day of bleeding, even if a POP has already been taken on that day. Additional contraceptive precautions are not then required. The remaining POPs should be discarded.
Postpartum and post-abortum use: After pregnancy, GINETTE 35 can be started 21 days after a vaginal delivery, provided that the patient is fully ambulant and there are no puerperal complications. Additional contraceptive precautions will be required for the first 7 days of pill taking. Since the first postpartum ovulation may precede the first bleeding, another method of contraception should be used in the interval between childbirth and the first course of tablets. Lactation is contraindicated with GINETTE 35. After a first trimester abortion, GINETTE 35 may be started immediately, in which case no additional contraceptive precautions are required.
Special Circumstances Requiring Additional Contraception
Incorrect administration: A single delayed tablet should be taken as soon as possible, and if this can be done within 12 hours of the correct time, contraceptive protection is maintained. With longer delays, additional contraception is needed. Only the most recently delayed tablet should be taken, earlier missed tablets being omitted, and additional non-hormonal methods of contraception (except the rhythm or temperature methods) should be used for the next 7 days, while the next 7 tablets are being taken. Additionally, therefore, if tablet(s) have been missed during the last 7 days of a pack, there should be no break before the next pack is started. In this situation, a withdrawal bleed should not be expected until the end of the second pack. Some breakthrough bleeding may occur on the days a tablet is taken, but this is not clinically significant. If the patient does not have a withdrawal bleed during the tablet-free interval following the end of the second pack, the possibility of pregnancy must be ruled out before starting the next pack.
Gastrointestinal upset: Vomiting or diarrhoea may reduce the efficacy of oral contraceptives by preventing full absorption, but even so, tablets from the current pack should be continued. Additional non-hormonal methods of contraception (except the rhythm or temperature methods) should be used during the gastrointestinal upset and for 7 days following the upset. If these 7 days overrun the end of a pack, the next pack should be started without a break. In this situation, a withdrawal bleed should not be expected until the end of the second pack. If the patient does not have a withdrawal bleed during the tablet-free interval following the end of the second pack, the possibility of pregnancy must be ruled out before starting the next pack. Other methods of contraception should be considered if the gastrointestinal disorder is likely to be prolonged.
- Pregnancy or lactation
- Severe disturbances of liver function, jaundice or persistent itching during a previous pregnancy, Dubin-Johnson syndrome, Rotor syndrome, previous or existing liver tumours
- Personal or family history of confirmed, idiopathic venous thromboembolism (VTE) (where a family history refers to VTE in a sibling or parent at a relatively early age)
- Current venous thrombotic or embolic processes
- Existing or previous arterial thrombotic or embolic processes
- The presence of a severe or multiple risk factor(s) for venous or arterial thrombosis
- Sickle-cell anaemia
- Mammary or endometrial carcinoma, or a history of these conditions
- Severe diabetes mellitus with vascular changes
- Disorders of lipid metabolism
- History of herpes gestationis
- Deterioration of otosclerosis during pregnancy
- Undiagnosed abnormal vaginal bleeding
- Hypersensitivity to any of the components of GINETTE 35
General
In animal studies, CPA-EE (like many other steroids), when given in very high doses and for the majority of the life span, has been found to cause an increase in the incidence of tumours, including carcinoma, in the liver of rats. The relevance of this finding to humans is unknown.
In rare cases, benign and, in even rarer cases, malignant liver tumours, leading in isolated cases to life-threatening intra-abdominal haemorrhage, have been observed after the use of hormonal substances such as cyproterone and ethinylestradiol. If severe upper abdominal complaints, liver enlargement or signs of intra-abdominal haemorrhage occur, a liver tumour should be included in the differential diagnosis.
Animal studies have revealed that the feminization of male foetuses may occur if cyproterone acetate is administered during the phase of embryogenesis at which differentiation of the external genitalia occurs. Although the results of these tests are not necessarily relevant to humans, the possibility must be considered that administration of CPA-EE to women after the 45th day of pregnancy could cause the feminization of male foetuses. It follows from this that pregnancy is an absolute contraindication for treatment with GINETTE 35, and must be excluded before such treatment is begun.
GINETTE 35 is composed of the progestogen, cyproterone acetate, and the oestrogen, ethinylestradiol, and is administered for 21 days of a monthly cycle. It, therefore, has a similar composition to that of a combined oral contraceptive (COC). The use of any COC or CPA-EE carries an increased risk for VTE, including deep venous thrombosis and pulmonary embolism, compared with no use. The excess risk of VTE is highest during the first year a woman ever uses a COC. This increased risk is less than the risk of VTE associated with pregnancy, which is estimated as 60 per 100,000 pregnancies.
Full recovery from such disorders does not always occur; VTE is fatal in 1-2% of cases. Epidemiological studies have shown that the incidence of VTE in users of oral contraceptives with low oestrogen content (<50 μg ethinylestradiol) is up to 40 cases per 1,00,000 women- years. This compares with 5-10 cases per 1,00,000 women-years for non-users. Certain factors may increase the risk of venous thrombosis, e.g., severe obesity (body mass index >30 kg/m2), increasing age, a genetic predisposition to clotting or a personal or family history of confirmed idiopathic VTE. In addition, the risk of VTE may be temporarily increased by prolonged immobilization, major surgery, any surgery to the legs, or major trauma.
There is some epidemiological evidence that the incidence of VTE is higher in users of CPA- EE when compared to users of COCs with low oestrogen content (<50μg).
The user group of CPA-EE as a treatment for severe acne or moderately severe hirsutism is likely to include patients that may have an inherently increased cardiovascular risk such as that associated with polycystic ovarian syndrome.
Epidemiological studies have also associated the use of COCs with an increased risk for arterial (myocardial infarction, transient ischaemic attack) thromboembolism. Certain factors such as smoking, obesity, cardiovascular disease, hypertension, diabetes and migraine may increase the risk of arterial thromboembolism. The risk of arterial thrombosis associated with oral contraceptives increases with age, and this risk is aggravated by cigarette smoking.
Numerous epidemiological studies have been reported on the risks of ovarian, endometrial, cervical and breast cancer in women using COCs. The evidence is clear that COCs offer substantial protection against both ovarian and endometrial cancer.
An increased risk of cervical cancer in long-term users of COCs has been reported in some studies, but there continues to be controversy about the extent to which this is attributable to the confounding effects of sexual behaviour and other factors.
Breast cancer is rare among women under 40 years of age whether or not they take COCs. Whilst this background risk increases with age, the excess number of breast cancer diagnoses in current and recent COC users is small in relation to the overall risk of breast cancer.
The most important risk factor for breast cancer in COC users is the age at which women discontinue the COC; the older the age at the time of discontinuation, the more breast cancers are diagnosed. Duration of use is less important and the excess risk gradually disappears during the course of the 10 years after stopping COC use such that, by 10 years, there appears to be no excess.
The possible increase in the risk of breast cancer should be discussed with the user and weighed against the benefits of COCs, taking into account the evidence that they offer substantial protection against the risk of developing certain other cancers (e.g., ovarian and endometrial cancer).
Reasons for Stopping Ginette 35 Immediately
- Occurrence for the first time, or exacerbation, of migraine headaches or unusually frequent or unusually severe headaches.
- Sudden disturbances of vision or hearing or other perceptual disorders.
- First signs of thrombophlebitis or thromboembolic symptoms (e.g., unusual pains in or swelling of the leg(s), stabbing pains on breathing or coughing for no apparent reason). Feeling of pain and tightness in the chest.
- Six weeks before an elective major operation (e.g., abdominal, orthopaedic), or any surgery to the legs, medical treatment for varicose veins, or prolonged immobilization, e.g., after accidents or surgery. Do not restart until two weeks after full ambulation. In case of emergency surgery, thrombotic prophylaxis is usually indicated e.g., subcutaneous heparin.
- Onset of jaundice, hepatitis, itching of the whole body.
- Increase in epileptic seizures.
- Significant rise in blood pressure.
- Onset of severe depression.
- Severe upper abdominal pain or liver enlargement.
- Clear worsening of conditions known to deteriorate during the use of hormonal contraception or during pregnancy.
- Pregnancy is a reason for stopping immediately because it has been suggested by some investigations that oral contraceptives taken in early pregnancy may slightly increase the risk of foetal malformations. Other investigations have failed to support these findings. The possibility, therefore, cannot be excluded, but it is certain that if a risk exists at all, it is small.
For each woman, assessment should be made prior to starting oral contraceptives (and at regular intervals thereafter) and should include a personal and family medical history. Physical examination should be guided by this and by the contraindications and warnings for this product. The frequency and nature of these assessments should be based upon relevant guidelines and should be adapted to the individual woman, but should include measurement of blood pressure and, if judged appropriate by the clinician, a breast, abdominal and pelvic examination including, cervical cytology.
The possibility cannot be ruled out that certain chronic diseases may occasionally deteriorate during the use of CPA-EE. The following conditions require strict medical supervision during medication with oral contraceptives. Deterioration or first appearance of any of these conditions may indicate that GINETTE 35 should be discontinued:
- Diabetes mellitus, or a tendency towards diabetes mellitus (e.g., unexplained glycosuria), hypertension, varicose veins, a history of phlebitis, otosclerosis, multiple sclerosis, epilepsy, porphyria, tetany, disturbed liver function, Sydenham's chorea, renal dysfunction, family history of clotting disorders, obesity, family history of breast cancer and patient history of benign breast disease, clinical depression, systemic lupus erythematosus, uterine fibroids, an intolerance to contact lenses, migraine, gall stones, cardiovascular diseases, chloasma, asthma, or any disease that is prone to worsen during pregnancy.
- Patients with a history of depression or any condition mentioned above should be monitored during treatment with GINETTE 35.
- If GINETTE 35 is discontinued, other methods of contraception should be introduced if needed.
- It should be borne in mind that the use of ultraviolet lamps for the treatment of acne or prolonged exposure to sunlight increases the risk of the deterioration of chloasma.
- Some women may experience amenorrhoea or oligomenorrhoea after the discontinuation of GINETTE 35, especially when these conditions existed prior to use. Women should be informed of this possibility.
Drug Interactions
Hepatic enzyme inducers such as barbiturates, primidone, phenobarbitone, phenytoin, phenylbutazone, rifampicin, carbamazepine and griseofulvin can impair the contraceptive efficacy of GINETTE 35. For women receiving long-term therapy with hepatic enzyme inducers, another method of contraception should be used. The use of antibiotics may also reduce the contraceptive efficacy of GINETTE 35, possibly by altering the intestinal flora.
Women receiving short courses of enzyme inducers and broad-spectrum antibiotics should take additional, non-hormonal (except the rhythm or temperature methods) contraceptive precautions during the time of concurrent medication and for 7 days afterwards. If these 7 days overrun the end of a pack, the next pack should be started without a break. In this situation, a withdrawal bleed should not be expected until the end of the second pack. If the patient does not have a withdrawal bleed during the 7 tablet-free days, the possibility of pregnancy must be ruled out before resuming with the next pack.
The possibility cannot be ruled out that oral tetracyclines, if used in conjunction with GINETTE 35 may reduce its contraceptive efficacy, although it has not been shown. When drugs of these classes are being taken it is therefore, advisable to use additional non- hormonal methods of contraception (except the rhythm or temperature methods) since an extremely high degree of protection must be provided when GINETTE 35 is being taken. With rifampicin, additional contraceptive precautions should be continued for four weeks after the treatment stops, even if only a short course was administered.
The requirement for oral antidiabetics or insulin can change as a result of the effect on glucose tolerance.
The herbal remedy, St. John's wort (Hypericum perforatum), should not be taken concomitantly with GINETTE 35 as this could potentially lead to a loss of the contraceptive effect.
Pregnancy
Contraindicated.
Animal studies have revealed that the feminization of male foetuses may occur if cyproterone acetate is administered during the phase of embryogenesis at which differentiation of the external genitalia occurs. Although the results of these tests are not necessarily relevant to humans, the possibility must be considered that the administration of CPA-EE to women after the 45th day of pregnancy could cause feminization of male foetuses. It follows from this that pregnancy is an absolute contraindication for treatment with GINETTE 35 and must be excluded before such treatment is begun.
Lactation
Undesirable Effects
There is an increased risk of VTE for all women who use CPA-EE.
In rare cases, headache, gastric upset, nausea, vomiting, breast tenderness, changes in body weight, changes in libido, and depressive moods can occur.
Post marketing reports of severe depression in patients using CPA-EE have been received. However, a causal relationship between clinical depression and CPA-EE has not been established.
In predisposed women, the use of CPA-EE can sometimes cause chloasma, which is exacerbated by exposure to sunlight. Such women should avoid prolonged exposure to sunlight.
Individual cases of poor tolerance of contact lenses have been reported with the use of oral contraceptives. Contact lens wearers who develop changes in lens tolerance should be assessed by an ophthalmologist.
Menstrual Changes
Reduction of menstrual flow: This is not abnormal and it is to be expected in some patients. Indeed, it may be beneficial where heavy periods were previously experienced.
Missed menstruation: Occasionally, withdrawal bleeding may not occur at all. If the tablets have been taken correctly, pregnancy is unlikely. Should bleeding fail to occur during the tablet-free interval, the possibility of pregnancy must be excluded before the next pack is started.
Intermenstrual bleeding: "Spotting" or heavier "breakthrough bleeding" sometimes occurs during the time when the tablets are being taken, especially in the first few cycles, and normally cease spontaneously. GINETTE 35 should, therefore, be continued even if irregular bleeding occurs. If irregular bleeding is persistent, appropriate diagnostic measures to exclude an organic cause are indicated and may include curettage. This also applies in the case of spotting, which occurs at regular intervals in several consecutive cycles or which occurs for the first time after long use of CPA-EE.
Effect on Blood Chemistry
The use of oral contraceptives may influence the results of certain laboratory tests including biochemical parameters of liver, thyroid, adrenal and renal function, plasma levels of carrier proteins and lipid/lipoprotein fractions, parameters of carbohydrate metabolism and parameters of coagulation and fibrinolysis. Laboratory staff should, therefore, be informed about oral contraceptive use when laboratory tests are requested.
Overdose may cause nausea, vomiting and withdrawal bleeding. There are no specific antidotes and further treatment should be symptomatic.
Store in a cool, dry place. Protect from moisture.
GINETTE 35 is available in a blister pack of 21 tablets.
GINETTE 35 is a hormonal tablet containing two hormones - cyproterone acetate (2 mg) and ethinylestradiol (0.035 mg).
GINETTE 35 should be taken at the same time each day, preferably after the evening meal or at bedtime.
You should begin GINETTE 35 on the first day (day 1) of your menstrual period or on the day instructed by your doctor and continue taking the tablets, following the arrows, for 21 consecutive days. Following the 21 days, no tablets have to be taken for the next 7 days (tablet-free days). Withdrawal bleeding (menses) could occur during these 7 tablet-free days.
A new pack of GINETTE 35 is started by taking the tablets on the day after 7 tablet-free days. To remember to start the next pack of GINETTE 35, mark the day you have to start the next pack on the calendar or set an alarm on your mobile.
Yes. For GINETTE 35 to be effective, it has to be taken daily at a time convenient to you.
It does not matter at what time of the day the tablet is taken, but once selected, the tablet should be taken as near as possible at the same time each day.
Tips to remember to take GINETTE 35 regularly:
- Carry GINETTE 35 in your purse/handbag.
- Keep a spare pack of GINETTE 35.
- Keep GINETTE 35 at a place where you can see it, e.g., next to your toothbrush.
- Link the time of taking GINETTE 35 to a daily activity, e.g., before going to bed at night, or after dinner.
There is no need to worry. If you miss a tablet and it is -
-
Less than 12 hours, take the tablet as soon as you remember and take that day's tablet at the usual time. This way you will be taking two tablets on that day.
-
More than 12 hours, discard that tablet and continue taking the remaining tablets according to the schedule. If you are using GINETTE 35 and are sexually active, use a barrier method of contraception also (e.g., condom) for the next 7 days.
1. The Contraception Report 2001;12:4-7
2. BMJ 1998;317:329-32
3. Endocrinology and Metabolism Clinics of North America 1998;27:877-902
4. The Lancet 2003;361:1894-901
5. Skin Therapy Letter 1999;4:1-8
6. Diane-35 Canadian product monograph
7. Clinical Trial Journal 1990;27(6):392-402
8. Skin Therapy Letter 2001;6:1-8
9. British Journal of Dermatology 1988;118:95-99
10. Clinical Endocrinology 2002;57:231-234
11. Acta Obstet Gynecol Scand 1990;69:61-63
12. Clinical and Experimental Dermatology 1987;12:319
13. Human Reproduction 2002;17(7):1729-1737




























