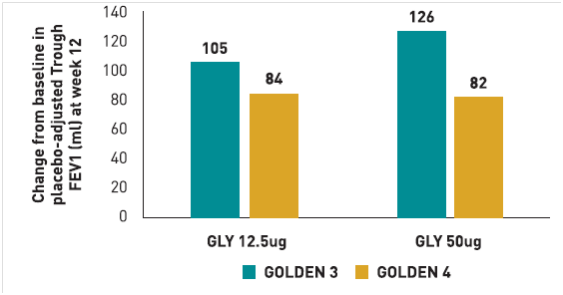Glycohale - F Product Monograph
Role of the LABA-LAMA Combination in COPD Management
COPD is a chronic respiratory disease characterized by non-reversible airflow obstruction due to structural changes in the airways, caused by an inflammatory response secondary to inhalation of noxious particles (such as cigarette smoke, biomass fuel and air pollution).1 This in turn may contribute to ventilation-perfusion mismatch, worsened gas exchange, dyspnea, inactivity and disease exacerbations. 5
Bronchodilation represents the cornerstone of the pharmacological treatment of COPD. Long-acting bronchodilators, whether β2 agonists (LABAs) or muscarinic antagonists (LAMAs), are central to symptom management in patients with COPD. 6
LAMAs and LABAs Achieve Bronchodilation through Different Mechanisms: 6,7
- Muscarinic antagonists block acetylcholine-mediated bronchoconstriction by binding to M3 receptors in airway smooth muscle
- β2 agonists induce smooth muscle relaxation by stimulating β2-adrenergic receptors.
Both classes of drugs have been shown to not only improve lung function and health-related quality of life (HRQoL), but also prevent exacerbations and increase exercise endurance by reducing pulmonary hyperinflation and dyspnea.6,7
Combining different classes of drugs and optimizing the effectiveness of inhalation therapy may reduce the doses of each medication while decreasing the risk of side effects. 6
Accumulating evidence from randomized controlled trials (RCTs) has shown that LABA/LAMA fixed-dose combinations (FDCs) have beneficial effects on lung function and patient-reported outcomes compared with LAMA or LABA/ICS treatments, while demonstrating a similar safety profile.6
One recent meta-analysis by Rodrigo et al including eighteen studies (23 trials) (N=20,185) reported greater efficacy and comparable safety profiles observed with LABA/LAMA combinations versus LAMA or LABA/ICS. 6
Results of the meta-analysis:
- LABA/LAMA significantly improved trough forced expiratory volume in 1 second (FEV1) from baseline to week 12 versus both LAMA and LABA/ICS (0.07 L and 0.08 L, P<0.0001) with patients more likely to achieve clinically important improvements in FEV1 of >100 mL (risk ratio [RR]: 1.33, 95% confidence interval [CI]: [1.20, 1.46] and RR: 1.44, 95% CI: [1.33, 1.56], respectively).
- LABA/LAMA improved transitional dyspnea index and St George’s Respiratory Questionnaire scores at week 12 versus LAMA (both P<0.0001), but not versus LABA/ICS
- LABA/LAMA use reduced rescue medication use versus both (P<0.0001 and P=0.001, respectively).
- LABA/LAMA significantly reduced moderate/severe exacerbation rate compared with LABA/ICS (RR 0.82, 95% CI: [0.75, 0.91]).
- Adverse event (AE) incidence was no different for LABA/LAMA versus LAMA treatment, but it was lower versus LABA/ICS (RR 0.94, 95% CI: [0.89, 0.99]), including a lower pneumonia risk (RR 0.59, 95% CI: [0.43, 0.81]).
- LABA/LAMA presented a lower risk for withdrawals due to lack of efficacy versus LAMA (RR: 0.66, 95% CI: [0.51, 0.87]) and due to AEs versus LABA/ICS (RR: 0.83, 95% CI: [0.69, 0.99]).
|
Similar results were reported in the Cochrane Review 8 which concluded that For the treatment of COPD, LAMA+LABA vs ICS+LABA results in:
|
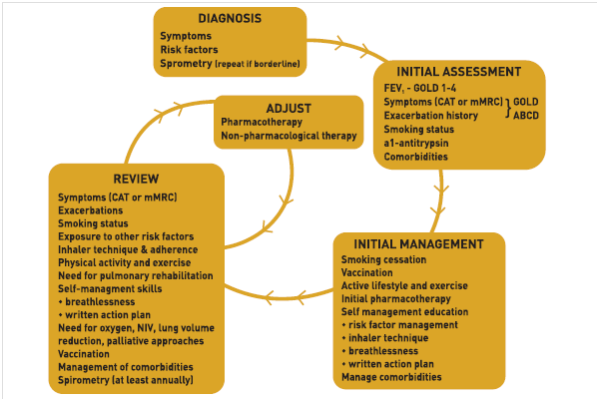
The Gold Guidelines Recommendations
The GOLD 2019 guidelines recommend combining two bronchodilators with different mechanisms of action over increasing the dose of a single bronchodilator for the management of COPD to increase the degree of bronchodilation with a lower risk of side effects.
For Group D Patients
For patients with more severe symptoms (order of magnitude of CAT™ > 20), especially driven by greater dyspnea and / or exercise limitation, LAMA/LABA may be chosen as initial treatment (Fig 1). 1
Follow Up Pharmacological Treatment 1
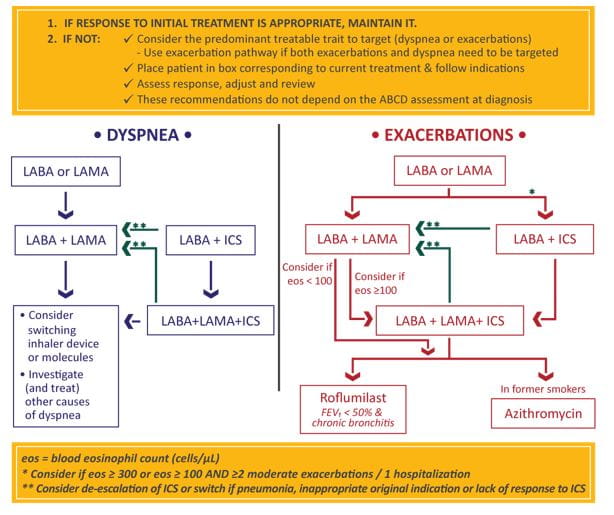
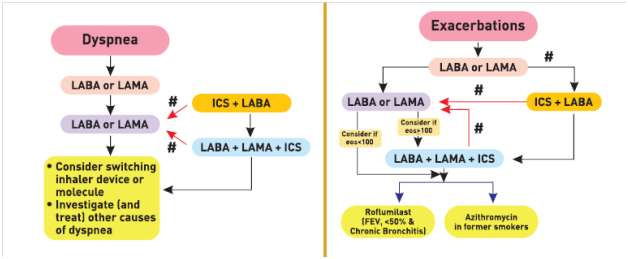
The GOLD guidelines provide a separate algorithm for follow-up treatment based on symptoms and exacerbations and are designed to facilitate management of patients taking maintenance treatment(s), whether early after initial treatment or after years of follow-up. These recommendations do not depend on the patient’s GOLD group at diagnosis, but can be applied to any patient who is already taking maintenance treatment(s). The algorithm suggests that the need to treat primarily dyspnea/exercise limitation or prevent exacerbations further should be evaluated. If a change in treatment is considered necessary, then the corresponding algorithm for dyspnea (Figure 2 left column) or exacerbations (Figure 2 right column) should be selected. 1
Glycopyrronium/ formoterol (25/12 mcg): Pharmacology
Pharmacologic effects of Formoterol in COPD
The pharmacologic effects of β2 -agonist formoterol is partly attributed to its ability to activate intracellular adenylyl cyclase, which stimulates the conversion of ATP to cAMP in bronchial smooth muscle cells. Increased cAMP causes relaxation of the smooth muscle and results in bronchodilatation. 9
Formoterol is moderately lipophilic, and therefore penetrates and is stored in smooth-muscle cell membranes, resulting in a long duration of bronchodilatory action. The hydrophilic properties of formoterol enable it to access and activate the β 2 -receptor rapidly and exert a rapid onset of action 9. (Fig 3)
Pharmacokinetics
The pharmacokinetics of formoterol are summarised in Table 1 .
|
Absorption |
As with other inhalers, the majority of inhaled formoterol fumarate delivered may be swallowed and then absorbed from the gastrointestinal tract In healthy volunteers: Maximum plasma drug concentration (after a single dose of 120 mcg): 92 pg/ mL Mean plasma concentration (after 12 weeks treatment with 12 and 24 mcg BID): 4.0- 8.8 pg/ mL and 8.0- 17.3 pg/ mL In COPD patients Formoterol 12 or 24 mcg twice daily as oral inhalation for 12 weeks, had an accumulation index of 1.19 - 1.38 (based on urinary excretion of unchanged formoterol suggesting some accumulation in plasma with multiple dosing. The excreted amounts of formoterol at steady-state were close to those predicted based on single-dose kinetics. |
|
Distribution |
Binding to human plasma proteins (in vitro): 61- 64 % (concentrations: 0.1-100 ng/mL) Binding to human serum albumin (in vitro): 31-38 % (concentration range: 5- 500 ng/ mL) |
|
Metabolism |
Metabolized primarily by direct glucuronidation at both the phenolic or aliphatic hydroxyl group and O-demethylation followed by glucuronide conjugation at either phenolic hydroxyl groups. The most prominent pathway involves direct conjugation at the phenolic hydroxyl group. The second major pathway involves O-demethylation followed by conjugation at the phenolic 2'-hydroxyl group. Four cytochrome P450 isozymes (CYP2D6, CYP2C19, CYP2C9 and CYP2A6) are involved in the O-demethylation of formoterol. Minor pathways involve sulfate conjugation of formoterol and deformylation followed by sulfate conjugation. Formoterol did not inhibit CYP450 enzymes at therapeutically relevant concentrations. Some patients may be deficient in CYP2D6 or 2C19 or both. Whether a deficiency in one or both of these isozymes results in elevated systemic exposure to formoterol or systemic adverse effects has not been adequately explored. |
|
Elimination |
Following oral administration of 80 mcg of radiolabeled formoterol fumarate to 2 healthy subjects, 59%-62% of the radioactivity was eliminated in the urine and 32%-34% in the faeces over a period of 104 hours. Renal clearance of formoterol from blood in these subjects was about 150 mL/min. Following inhalation of a 12 mcg or 24 mcg dose by 16 patients with asthma, about 10% and 15%-18% of the total dose was excreted in the urine as unchanged formoterol and direct conjugates of formoterol, respectively. Following inhalation of 12 mcg or 24 mcg dose by 18 patients with COPD the corresponding values were 7% and 6-9% of the dose, respectively. Based on plasma concentrations measured following inhalation of a single 120 mcg dose by 12 healthy subjects, the mean terminal elimination half-life was determined to be 10 hours. From urinary excretion rates measured in these subjects, the mean terminal elimination half-lives for the (R,R)- and (S,S)-enantiomers were determined to be 13.9 and 12.3 hours, respectively. The (R,R)- and (S,S)-enantiomers represented about 40% and 60% of unchanged drug excreted in the urine, respectively, following single inhaled doses between 12 and 120 mcg in healthy volunteers and single and repeated doses of 12 and 24 mcg in patients with asthma. Thus, the relative proportion of the two enantiomers remained constant over the dose range studied and there was no evidence of relative accumulation of one enantiomer over the other after repeated dosing. |
Pharmacologic Effects of Glycopyrronium in COPD
Glycopyrronium bromide, a quaternary ammonium compound, is a competitive muscarinic receptor antagonist that bronchodilates the airways by inhibiting acetylcholine induced bronchoconstriction in bronchial smooth muscle cells. 11 Glycopyrronium bromide has low oral bioavailability, and hence lower risk of systemic side effects due to absorption.12
In vitro, glycopyrronium bromide binds with high affinity to the M1, M2 and M3 receptors, and has 4- to 5-fold higher selectivity for human M1 and M3 receptors than for the human M2 receptor (equilibrium binding affinity constants of 9.60–9.81 and 9.47–9.64 vs. 8.70–9.25. (M3 receptor is the prime mediator of cholinergic bronchoconstriction). In addition, glycopyrronium bromide dissociates slower from the M3 and M1 receptors than from the M2 receptor (dissociation half-life 11.4 and 13.9 vs. 1.07 min; kinetic off rate 0.061 and 0.05 vs. 0.646 per min. (M2 receptor protects against bronchoconstriction, and has a role in cardiac function) 11
As compared to tiotropium: 13
- Glycopyrronium bromide had a 2.5- to 4.8-fold faster onset of action (5 minutes for glycopyrronium versus 30 minutes for tiotropium).
- Glycopyrronium bromide has the potential for an improved therapeutic index with a greater equilibrium binding selectivity (M3 selectivity ratio [ratio of the affinity constant for the M3 receptor vs. that for the M2 receptor] of 7.76-fold vs. 2.09-fold) and kinetic selectivity (M3 kinetic selectivity ratio [ratio of the area under the simulated association and dissociation curves for the M3 receptor vs. that for the M2 receptor] of 11.41-fold vs. 4.30-fold) for M3 versus M2
Glycopyrronium bromide is also more potent than ipratropium bromide and/or tiotropium bromide in terms of the concentration or -log molar concentration required to inhibit the contractile response by 50 % .11
|
Glycopyrronium in comparison with Tiotropium
|
Pharmacokinetics
The pharmacokinetics of glycopyrrolate are summarised in Table 2
|
Absorption |
|
|
Distribution |
After intravenous administration, the steady-state volume of distribution of glycopyrrolate was 83 L and the volume of distribution in the terminal phase was 376 L. The in vitro human plasma protein binding of glycopyrrolate was 38% to 41% at concentrations of 1 to 10 ng/mL |
|
Metabolism |
Metabolised into many mono- and bis-hydroxylated metabolites and direct hydrolysis resulting in formation of carboxylic acid derivative (M9). Multiple CYP isoenzymes contribute to the oxidative biotransformation of glycopyrrolate Hydrolysis to M9 is likely to be catalyzed by members from the cholinesterase family pre-systemically and/or via first pass metabolism from the swallowed dose fraction of orally inhaled glycopyrrolate. Glucuronide and/or sulfate conjugates of glycopyrrolate were found in urine of humans after repeated inhalation, accounting for about 3% of the dose. |
|
Elimination |
60-70 % of glycopyrronium is excreted renally and most of the metabolites show biliary clearance. Glycopyrrolate plasma concentrations declined in a multiphasic manner. The mean terminal elimination half-life was much longer after inhalation (33 to 53 hours) than after intravenous (6.2 hours) and oral (2.8 hours) administration. |
Clinical Efficacy
Clinical Efficacy of Formoterol 12 mcg bid
Randomized, controlled studies in patients with COPD have demonstrated that formoterol has a faster onset of action than other long-acting bronchodilators and that the effects of formoterol are sustained over 24 h with twice-daily dosing. These studies also suggest that formoterol in combination with bronchodilators with different mechanism of action is effective and safe for maintenance therapy in patients with moderate to severe COPD.
Dose Ranging Studies
In one of the early studies with formoterol, six-hundred and ninety-two COPD patients, with mean baseline forced expiratory volume in one second (FEV1) 54%, FEV1/forced vital capacity 75% of predicted, reversibility 6.4% pred, were treated with formoterol (6, 12 and 24 mcg b.i.d) or placebo via a Turbohaler for 12 weeks. 15
Results
- Formoterol (12 and 24 mcg) significantly reduced symptom scores for breathlessness (-7% and -9%, respectively) and chest tightness (-11% and -8%, respectively), reduced the need for rescue medication (-25% and -18%, respectively), and increased symptom free days (71% and 86%, respectively).
- FEV1 improved significantly after all three doses of formoterol (versus placebo).
- No unexpected adverse events were seen.
|
Further, in the year 2011, Bogdan et al. reported the efficacy and safety of inhaled formoterol at doses of 6 mcg and 12 mcg twice daily in patients with moderate-to-severe COPD (n=613). Similar to the previous report, this study demonstrated that there was no significant difference in the improvements in FEV1 values yielded by either dose of formoterol in comparison to placebo. However, the secondary outcomes such as improvement in SGRQ score of ≥ 4 (50.2 % for 6 mcg BID, p= 0.0682 v/s placebo and 59.2 % for 12 mcg BID, p=0.0004) and reduction in rescue inhaler use (-0.274 for 6 mcg BID, p=0.027 and -0.548 for 12 mcg BID, p <0.001) was significantly greater in the patients treated with 12 mcg BID as compared to those treated with 6 mcg BID, demonstrating the superiority of 12 mcg BID regimen of formoterol in management of COPD16.
Clinical Efficacy of Glycopyrronium 25 mcg bid
Inhaled glycopyrronium bromide is a LAMA approved in various countries, as a maintenance bronchodilator for the symptomatic treatment of adult patients with COPD. Several randomised controlled trials have endorsed the efficacy and safety of glycopyrronium in the maintenance of COPD patients.
Dose Ranging Studies
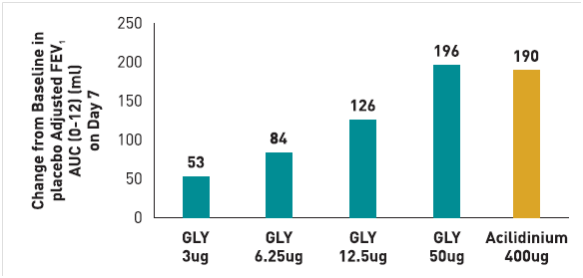
A randomized, double-blind, placebo-controlled study17 was carried out to determine the safety and efficacy of various doses of glycopyrronium in comparison to open-label active comparator tiotropium in patients with moderate-to-severe COPD (n=83). The primary endpoint in the study was mean trough FEV1 on day 7. Secondary endpoints included mean trough FEV1 on day 1 and post-dose FEV1 and FVC at individual time points post-dose. The results of the study (summarized in figure 4) indicated that
- 50 mcg was the minimum daily dose of glycopyrronium required to achieve statistically significant differences v/s placebo on day 7 that exceeded the MCID (> 120 mL), with a difference of 131 mL as compared to the placebo (p < 0.0001).
- The difference in trough FEV1 between tiotropium and placebo was 127 mL on day 7. Similar results were observed on day 1, where 50 mcg glycopyrronium demonstrated clinically relevant improvement in FEV1 versus placebo with a difference of 135 mL (p < 0.0001), the difference between tiotropium v/s placebo on day 1 was 112 mL (p < 0.0001).
- On day 1, the FEV1 values obtained after treatment with 50 mcg glycopyrronium was significantly greater than those achieved with tiotropium (p<0.05). Additionally, it was observed that the improvement in mean trough FEV1 with a daily dose of 50 mcg glycopyrronium was superior to the lower doses i.e. daily dose of 12.5 mcg (p<0.0001) and 25 mcg (p=0.0537) on day 7 and day 1 (p=0.0009 for 50 mcg v/s 25 mcg on day 1).
- The responder rates (number of patients showing ≥ 120 mL improvement in trough FEV1 relative to placebo) were higher in the group of patients receiving 50 mcg daily dose of glycopyrronium (61 %) as compared to 25 mcg (43 %) and 12.5 mcg (28%). (Fig 4)
All the doses of glycopyrronium used in the study were well-tolerated and most of the adverse events were transient and did not appear to be dose-related.
The results of the study clearly suggest that 50 mcg daily dose of glycopyrronium provides better bronchodilation as compared to the lower doses and may demonstrate rapid onset of action as compared to tiotropium, that may prove to be clinically beneficial for patients with COPD.
The results of the study clearly suggest that 50 mcg daily dose of glycopyrronium provides better bronchodilation as compared to the lower doses and may demonstrate rapid onset of action as compared to tiotropium, that may prove to be clinically beneficial for patients with COPD
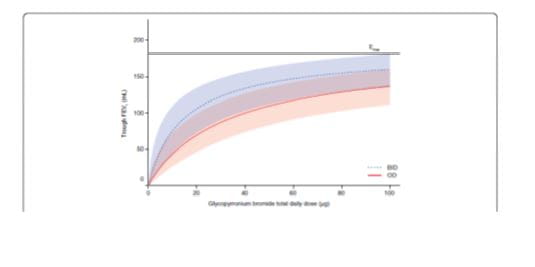
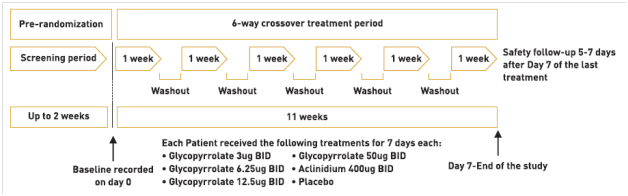
A study compared the efficacy and safety of glycopyrronium, as a once-daily (OD) regimen versus the same total dose, administered as a twice-daily (BID) regimen in patients with moderate-to-severe COPD. 18
In this double-blind, randomized, dose-finding trial with an eight-treatment, two-period, balanced incomplete block design, patients (smoking history ≥10 pack-years, post-bronchodilator FEV1 ≥30% and <80% predicted, FEV1/ FVC <0.7) were randomized to one of 16 independent sequences for 28 days. The primary endpoint was mean trough FEV1 at Day 28.
Results
385 patients (mean age 61.2 years; mean post-bronchodilator FEV1 53% predicted) were randomized; 88.6% completed.
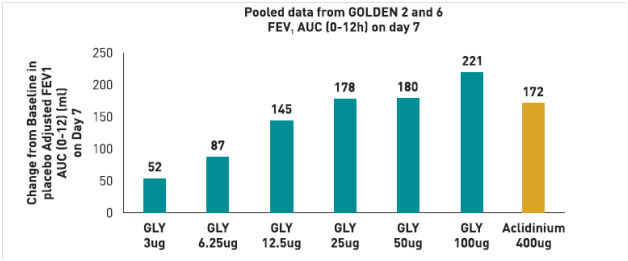
- All OD and BID dosing regimens produced dose-dependent bronchodilation; at Day 28, increases in mean trough FEV1 versus placebo were statistically significant for all regimens, ranging from 51 mL (glycopyrronium bromide 12.5 μg OD) to 160 mL (glycopyrronium bromide 50 μg BID).
- Pharmacodynamic steady state was reached by Day 7.
- There was a separation (≤37 mL) between BID and OD dose–response curves for mean trough FEV1 at steady-state in favour of BID dosing. (Fig 5)
- Over 24 hours, separation between OD and BID regimens was even smaller (FEV1 AUC0-24h maximum difference for equivalent daily dose regimens: 8 mL).
- Dose–response results for FEV1 at 12 hours, FEV1 AUC0-12h and FEV1 AUC0-4h at steady-state showed OD regimens provided greater improvement over placebo than BID regimens for total daily doses of 25 μg, 50 μg and 100 μg, while the reverse was true for OD versus BID regimens from 12–24 hours.
- Glycopyrronium bromide was safe and well tolerated at all doses.
|
Over the course of the 24-hour dosing period, differences between OD and BID regimens, for an equivalent daily dose, were negligible, and the dose–response relationship for AUC0-24h FEV1 was driven primarily by total daily dosage. The study further suggests that OD dosing may not offer additional benefits over BID dosing in all patients; some symptomatic patients may prefer more frequent relief with a BID regimen |
Clinical Efficacy of Glycopyrronium/ Formoterol: PINNACLE Studies `19-21
The PINNACLE clinical programme evaluated the efficacy (and safety) of glycopyrronium/formoterol(GFF MDI) twice daily in > 5000 patients with moderate to very severe COPD. The trials included patients aged 40–80 years with an established clinical history of moderate to very severe COPD (as defined by the American Thoracic Society/European Respiratory Society), who were current or former smokers (≥ 10 pack-years).
- PINNACLE-1 and -2 included patients from the USA, Australia and New Zealand
- PINNACLE-4 included patients from the USA, EU, Russia and Asia
- Patients completing 24 weeks’ active treatment in PINNACLE- 1 or -2 were randomly selected to participate in the extended 28-week PINNACLE-3 extension, where they continued to receive their assigned treatment.
Treatment /Dosage
- Eligible patients were randomized to receive twice daily glycopyrronium/formoterol 18/9.6 μg MDI(GFF MDI) , glycopyrronium 18 μg MDI (GP MDI) , formoterol 9.6 μg MDI (FF MDI) or placebo MDI in a double-blind fashion
- Some patients in PINNACLE-1 were also randomized to receive open-label tiotropium 18 μg dry powder inhaler (DPI) once daily.
Primary Efficacy
The primary efficacy endpoint was the least-squares mean (LSM) change from baseline in morning predose trough FEV1 at week 24 (PINNACLE-1, -2 and -4) or over 52 weeks (PINNACLE-3)
Results
Morning predose trough FEV1 at week 24
PINNACLE 1 and 2
In both studies, GFF MDI, GP MDI, and FF MDI showed significant improvements vs placebo MDI in change from baseline in morning predose trough FEV1 at week 24 (all P < .0001 in PINNACLE-1 and P < .02 in PINNACLE-2)
GFF MDI showed significant differences vs placebo MDI of 150 mL and 103 mL in PINNACLE-1 and PINNACLE-2, respectively (all P < .0001).
PINNACLE 1
In PINNACLE-1, GFF MDI showed significant differences of 59 mL and 64 mL vs GP MDI and FF MDI, respectively (all P < .0001).
PINNACLE 2
In PINNACLE-2, GFF MDI showed significant differences of 54 mL (P =.0003) and 56 mL (P = .0002) vs GP MDI and FF MDI, respectively.
The change from baseline in morning predose trough FEV1 over 24 weeks was similar but with slightly larger estimated differences vs placebo MDI (Fig 6).
A prospective subgroup analysis of the pooled ITT population showed that the benefits of GFF MDI on change from baseline in morning predose trough FEV1 over 24 weeks were consistent regardless of concomitant ICS use .
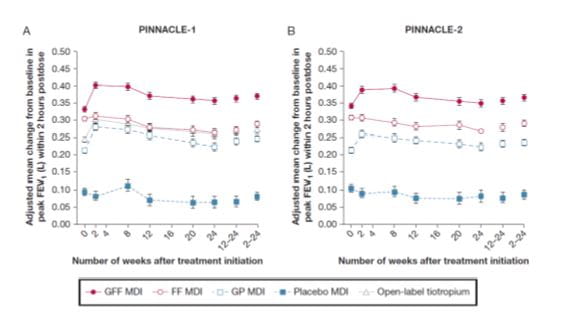
Change in FEV1 2 hours post dose
PINNACLE 1 and 2
For peak change from baseline in FEV1 within 2 hours postdose at week 24 , GFF MDI showed significant differences vs placebo and monocomponent MDIs in both PINNACLE-1 and PINNACLE-2 (all P < .0001).
The change from baseline in peak FEV1 within 2 hours postdose over 24 weeks was similar. (Fig 7).
Onset of action on Day 1
For onset of action on day 1 , GFF MDI showed a significant difference from placebo MDI at 5 min, which was the first time point assessed in both studies, with respective differences of 187 mL and 186 mL (all P < .0001).
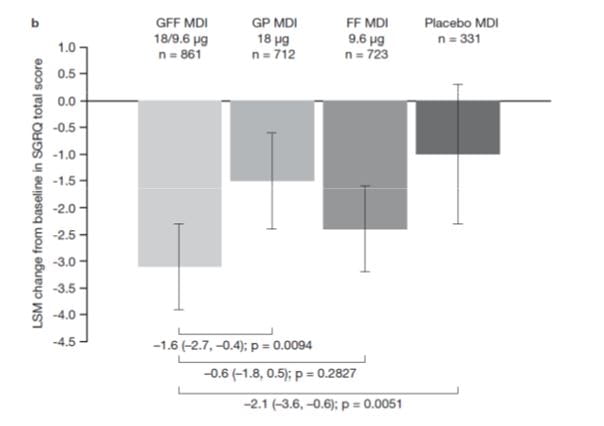
St. George’s Respiratory Questionnaire (SGRQ) scores
PINNACLE-1
In PINNACLE-1, GFF MDI showed significant differences in SGRQ total score at week 24 vs placebo (–2.52) and GP MDIs (–2.33).
GFFMDI-treated patients were more likely to achieve the minimum clinically important difference of 4 units in SGRQ total score vs GP MDI and placebo MDI in PINNACLE-1 (all P < .05). (Fig 8)
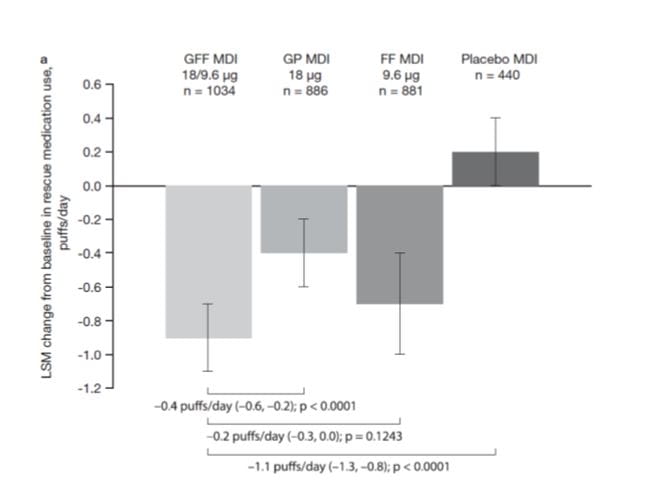
Use of rescue medication
PINNACLE 1 and 2
In PINNACLE-1 and PINNACLE-2, GFF MDI showed a significant reduction in rescue albuterol use over 24 weeks vs placebo MDI (–1.08 and –1.04 puffs/d, respectively).
In PINNACLE-2, GFF MDI also reduced rescue albuterol use compared with GP MDI (–0.57 puffs/day) and FF MDI (–0.29 puffs/day).
GP MDI and FF MDI reduced average daily rescue albuterol use over 24 weeks compared with placebo MDI in both studies.
Overall, the pooled data show that treatment with GFF MDI results in patients using appreciably less rescue medication than patients treated with placebo MDI or GP MDI (Figure 9).
PFT substudy
PINNACLE 1
In the 12-hour PFT substudy of PINNACLE-1, GFF MDI showed significant improvements in FEV1 AUC0-12 at week 12 vs placebo MDI, GP MDI, and FF MDI of 237 mL (P < .0001), 102 mL (P < .0001), and 56 mL (P = .0196), respectively.
PINNACLE 2
In the PINNACLE-2 PFT substudy, GFF MDI showed significant improvements in FEV1 AUC0-12 at week 12 vs placebo MDI, GP MDI, and FF MDI of 209 mL (P < .0001), 98 mL (P < .0001), and 67 mL, respectively (P = .0056).
Evening 12-hour postdose trough FEV1
PINNACLE 1 and 2
GFF MDI significantly improved evening 12-hour postdose trough FEV1 by 144 mL in PINNACLE-1 and by 158 mL in PINNACLE-2 vs placebo MDI (both P < .0001), with significant improvements vs GP MDI (61 mL; P = .0196) and FF MDI (65 mL; P = .0145) in PINNACLE-2.
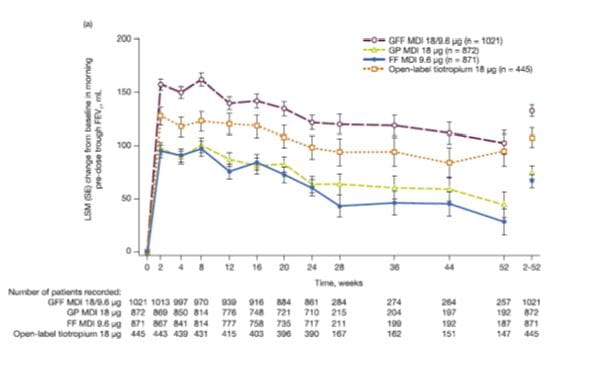
RESULTS OF PINNACLE 3
Change from baseline in morning pre-dose trough FEV1 over 52 weeks
For the primary efficacy endpoint of change from baseline in morning pre-dose trough FEV1 over 52 weeks, GFF MDI showed a significantly greater improvement from baseline compared with GP MDI (difference: 57 mL; p < 0.0001), FF MDI (difference: 65 mL; p < 0.0001) and open-label tiotropium (difference: 25 mL; p = 0.0117) (Fig. 10).
GP MDI demonstrated noninferiority to open-label tiotropium (difference: -31 mL; 95% CI: -52, -11) with the lower bound of the 95% CI greater than the pre-specified threshold for non-inferiority of -85 mL.
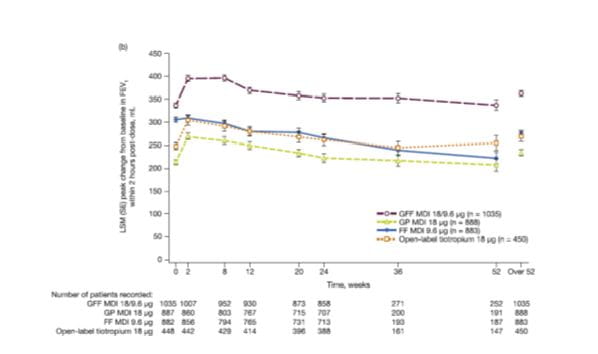
Peak change from baseline in FEV1 within 2 h post-dose over 52 weeks
For peak change from baseline in FEV1 within 2 h post-dose over 52 weeks, GFF MDI demonstrated significantly greater improvements compared with both monocomponents and open-label tiotropium (all p < 0.0001, Fig. 11,).
GP MDI demonstrated non-inferiority over 52 weeks versus open-label tiotropium (difference: -35 mL; 95% CI: -58, -13).
Symptoms and health-related quality of life (HRQoL)
For Self-Administered Computerized Transition Dyspnea Index (SAC-TDI) score over 52 weeks, GFF MDI showed statistically significant improvements compared with GP MDI (difference: 0.21; 95% CI: 0.07, 0.36; p = 0.0040) and FF MDI (difference: 0.16; 95% CI: 0.02, 0.31; p = 0.0273), with a numerical improvement versus open-label tiotropium (difference: 0.08; 95% CI: -0.10, 0.26; p = 0.3896).
GP MDI demonstrated non-inferiority over 52 weeks versus open-label tiotropium (difference: -0.14; 95% CI: -0.32,0.05).
Results for treatment differences in SAC-TDI focal score over 52 weeks are presented in Fig. 12
In the GFF MDI treatment arm, 24.9% of subjects achieved an improvement of -1.0 units in SAC-TDI score versus 22.9% for GP MDI, 21.7% for FF MDI and 23.2% for open-label tiotropium.
The odds of being a SAC-TDI responder were numerically higher for GFF MDI versus GP MDI (odds ratio [OR]: 1.12; p = 0.3065), FF MDI (OR:1.19; p ¼ 0.1013) and open-label tiotropium (OR: 1.09; p = 0.5167). The OR for GP MDI versus open-label tiotropium was 0.98 (95% CI: 0.74, 1.30).
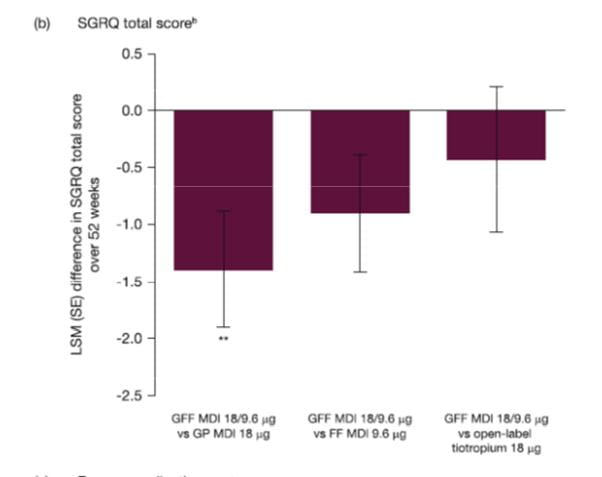
St. George’s Respiratory Questionnaire (SGRQ) scores
For change from baseline in SGRQ total score over 52 weeks, GFF MDI showed a significantly greater improvement compared with GP MDI (difference: -1.39; 95% CI: -2.38, -0.39; p = 0.0066), and numerical improvements versus FF MDI (difference: -0.90; 95% CI: -1.90, 0.10; p = 0.0764) and open-label tiotropium (difference: -0.43; 95% CI: -1.68, 0.82; p = 0.4989). GP MDI demonstrated non-inferiority over 52 weeks to open-label tiotropium (difference 0.95; 95% CI: -0.34, 2.25).
Results for treatment differences in SGRQ total score over 52 weeks are presented in Fig. 13.
In the GFF MDI treatment arm, 41.3% of subjects achieved the minimal clinically important difference of -4 units in SGRQ total score compared with 35.6% for GP MDI, 37.2% for FF MDI and 43.8% for open-label tiotropium. The odds of being an SGRQ responder were significantly greater for GFF MDI versus GP MDI (OR: 1.27; p = 0.0140), with a numerical improvement versus FF MDI (OR: 1.19; p = 0.0760), but not open-label tiotropium (OR: 0.90; p = 0.4100). The OR for GP MDI versus open-label tiotropium was 0.71 (95% CI: 0.55, 0.91).
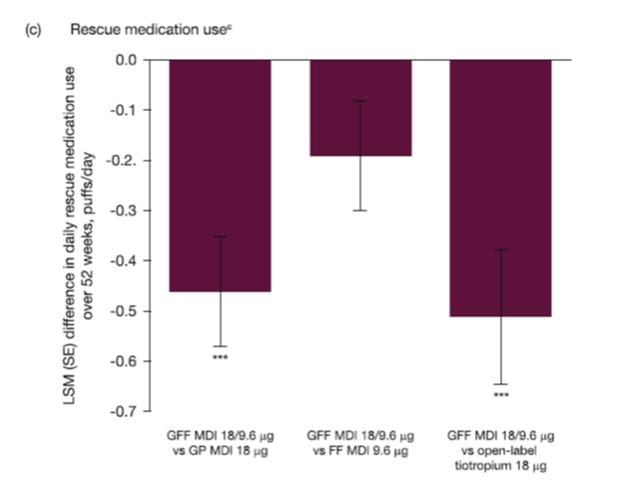
Rescue medication use
GFF MDI demonstrated a significantly greater reduction in average daily use of rescue salbutamol over 52 weeks compared with GP MDI (difference: -0.46 puffs/day; 95% CI: -0.67, -0.24; p < 0.0001) and open-label tiotropium (difference: -0.51 puffs/ day; 95% CI: -0.77, -0.24; p = 0.0002), with a numerical improvement versus FF MDI (-0.19 puffs/day; 95% CI: -0.41, 0.02; p = 0.0750). GP MDI demonstrated non-inferiority over 52 weeks versus open-label tiotropium with a treatment difference of -0.05 puffs/day (95% CI: -0.32, 0.22). Results for treatment differences in use of daily rescue medication over 52 weeks are presented in Fig. 14.
COPD exacerbations
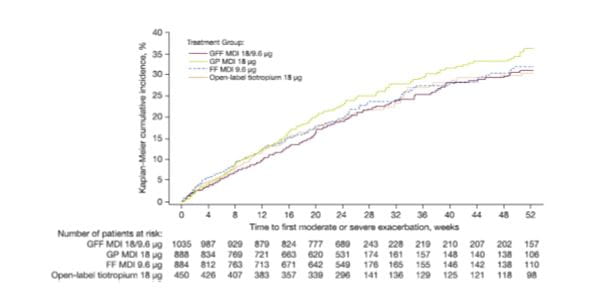
The percentage of subjects experiencing COPD exacerbations of any severity was 23.0%, 26.0%, 23.4% and 25.1% for GFF MDI, GP MDI, FF MDI and open-label tiotropium, respectively. The majority of exacerbations were moderate or severe, with a numerical improvement in model-estimated rate (per year) for GFF MDI versus both monocomponent MDIs. Treatment incidence rate ratios for GFF MDI versus GP MDI and FF MDI were 0.90 and 0.92, respectively. The time to first moderate or severe COPD exacerbation was generally longer for GFF MDI versus both monocomponent MDIs, and similar compared with open-label tiotropium (Fig. 15).
Clinical Efficacy of Glycopyrronium/Formoterol in Indian Patients 22
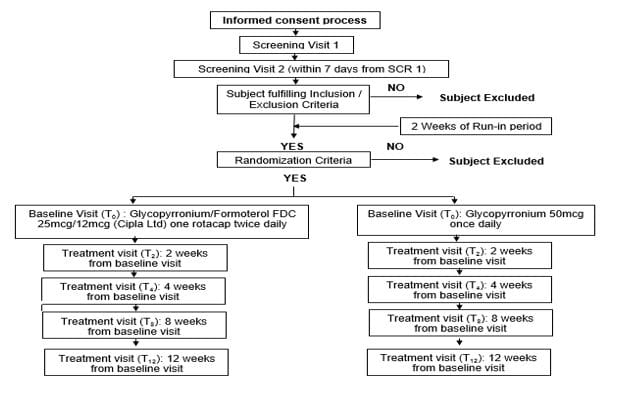
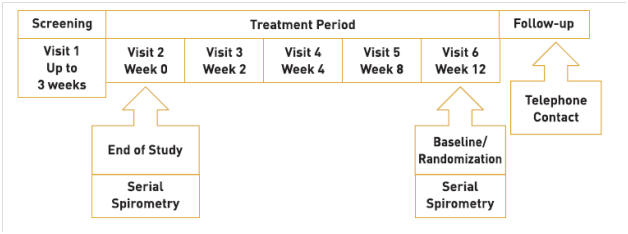
A randomized, prospective, open label, comparative, parallel group, multicentre 12 weeks’ study was conducted on Indian patients with COPD, with the following objectives:
- Primary Objective:
To demonstrate non-inferiority of Glycopyrronium/Formoterol FDC 25mcg/12mcg twice daily in comparison with Glycopyrronium 50mcg once daily in patients with moderate to severe COPD
- Secondary Objective:
To demonstrate superiority of Glycopyrronium/Formoterol FDC 25mcg/12mcg twice daily in comparison with Glycopyrronium 50mcg once daily in patients with moderate to severe COPD.
The study design is illustrated in Figure 16.
There was no statistically significant difference in the demographic and baseline characteristics (p>0.05) between the two treatment arms Glycopyrronium/Formoterol (25mcg/12mcg) and Glycopyrronium 50mcg. (Table 3)
|
Parameters (Mean± SD) |
Glycopyrronium/ Formoterol (N=158) |
Glycopyrronium (N=165) |
p-values |
|
Gender (M/F,%) |
150 (94.94%)/ 8 (5.06%) |
158 (95.76%)/ 7 (4.24%) |
0.726 |
|
Age (Yrs) |
56.53 ± 6.35 |
55.85 ± 6.56 |
0.3517 |
|
Duration of COPD (Yrs) |
4.11 ± 4.04 |
4.19 ± 4.19 |
0.8697 |
|
Smoking History |
23.20 ± 11.03 |
23.17 ± 11.94 |
0.9795 |
|
AEC |
246.91 ± 185.63 |
261.07 ± 193.40 |
0.5028 |
|
FEV1 Pre BD (L) |
1.21 ± 0.28 |
1.24 ± 0.30 |
0.4227 |
|
FVC Pre BD (L) |
2.20 ± 0.47 |
2.18 ± 0.51 |
0.6898 |
|
FEV1 Post BD (L) |
1.33 ± 0.28 |
1.33 ± 0.30 |
0.919 |
|
FVC Post BD (L) |
2.38 ± 0.49 |
2.34 ± 0.51 |
0.408 |
|
FEV1% of Predicted |
52.49 ± 9.03 |
52.05 ± 9.90 |
0.6779 |
|
Reversibility |
11.24 ± 16.54 |
8.68 ± 12.51 |
0.117 |
|
Pre Dose FEV1 |
1.20 ± 0.29 |
1.22 ± 0.31 |
0.4195 |
|
1 Hr Post Dose FEV1 |
1.35 ± 0.30 |
1.35 ± 0.36 |
0.9923 |
|
Pre Dose FVC |
2.17 ± 0.46 |
2.16 ± 0.49 |
0.8282 |
|
1 Hr Post Dose FVC |
2.37 ± 0.49 |
2.33 ± 0.54 |
0.398 |
|
mMRC Score |
2.04 ± 0.84 |
2.09 ± 0.74 |
0.5467 |
|
CAT Score |
20.68 ± 7.63 |
20.72 ± 7.66 |
0.9588 |
|
Serum Potassium |
4.42 ± 0.64 |
4.40 ± 0.60 |
0.7797 |
The results are presented below:
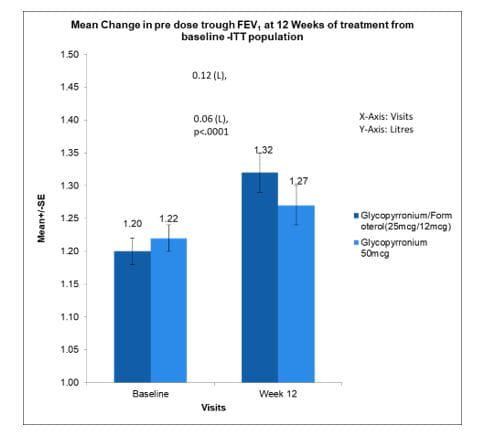
Mean change in pre-dose trough FEV1 at 12 weeks of treatment from baseline. (ITT Population)
The mean change in pre-dose trough FEV1 at 12 weeks of treatment from baseline within Glycopyrronium/Formoterol (25mcg/12mcg) group was 120ml (p<0.0001) while in Glycopyrronium 50mcg group was 60ml (p<0.0126).
There was a statistically significant difference of 60ml (p<0.0001) in mean change in pre-dose trough FEV1 at 12 weeks of treatment from baseline between Glycopyrronium/Formoterol (25mcg/12mcg) group vs. Glycopyrronium 50mcg group.(Fig 17)
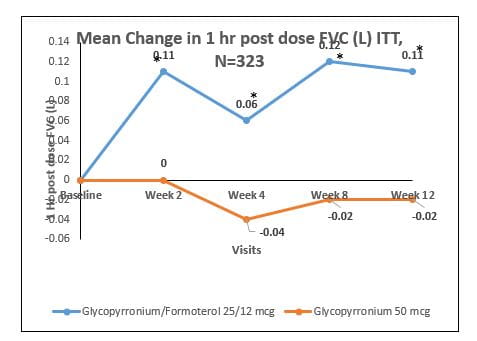
Mean change in 1 hour post dose FEV1 at 2, 4, 8 and 12 weeks of treatment from baseline (ITT Population).
The mean change in 1 hour post-dose FEV1 at 2, 4, 8 and 12 weeks of treatment from baseline within Glycopyrronium/Formoterol (25mcg/12mcg) group was 90ml (p=0.0026), 60ml (p=0.0131), 130ml (p<0.0001) and 100 ml (p=0.0003) respectively while in Glycopyrronium 50mcg was 0ml (p=0.8905), -20ml (p=0.3178), 20ml (p=0.4058) and 10ml (p=0.6846) respectively.
There was a statistically significant difference of 90ml (p<0.0001), 90ml (p<0.0001), 110ml (p<0.0001) and 80ml (p<0.0001) respectively in mean change in 1 hour post-dose FEV1 at 2, 4, 8 and 12 weeks of treatment from baseline in Glycopyrronium/Formoterol (25mcg/12mcg) group vs Glycopyrronium 50mcg group. (Fig 18)
Mean change in 1 hour post dose FVC at 2, 4, 8 and 12 weeks of treatment from baseline (ITT Population).
The mean change in 1 hour post-dose FVC at 2, 4, 8 and 12 weeks of treatment from baseline in Glycopyrronium/Formoterol (25mcg/12mcg) group was 110ml (p=0.0057), 60ml (p=0.0541), 120ml (p=0.0003) and 110 ml (p=0.0011) respectively while in Glycopyrronium 50mcg was 0ml (p=0.98888), -40ml (p=0.1935), -20ml (p=0.6087) and -20ml (p=0.4889) respectively.
There was a statistically significant difference of 110ml (p<0.0001), 100ml (p<0.0001), 140ml (p<0.0001) and 140ml (p<0.0001) respectively in mean change in 1 hour post-dose FVC at 2, 4, 8 and 12 weeks of treatment from baseline in Glycopyrronium/Formoterol (25mcg/12mcg) group vs Glycopyrronium 50mcg group. (Fig 19)
Mean change in pre-dose trough FVC at 2, 4, 8 and 12 weeks of treatment from baseline (ITT Population).
The mean change in pre-dose trough FVC at 2, 4, 8 and 12 weeks of treatment from baseline in Glycopyrronium/Formoterol (25mcg/12mcg) group was 90ml (p=0.0076), 110ml (p=0.0021), 140ml (p=0.0003) and 100 ml (p=0.0033) respectively while in Glycopyrronium 50mcg was 50ml (p=0.0776), 30ml (p=0.4076), 30ml (p=0.3252) and 40ml (p=0.2403) respectively.
There was a statistically significant difference of 30ml (p<0.0001), 80ml (p<0.0001), 110ml (p<0.0001) and 60ml (p<0.0001) respectively in mean change in pre-dose trough FVC at 2, 4, 8 and 12 weeks of treatment from baseline in Glycopyrronium/Formoterol (25mcg/12mcg) group vs. Glycopyrronium 50mcg group. (Fig 20)
Mean change in pre-dose trough FEV1 at 2, 4, and 8 weeks of treatment from baseline (ITT Population).
The mean change in pre-dose trough FEV1 at 2, 4 and 8 weeks of treatment from baseline in Glycopyrronium/Formoterol (25mcg/12mcg) group was 90ml (p<0.0001), 100ml (p<0.0001) and 160ml (p<0.0001) respectively while in Glycopyrronium 50mcg was 30ml (p=0.0254), 30ml (p=0.0309) and 70ml (p=0.0009) respectively.
There was a statistically significant difference in mean change of 60ml (p<0.0001), 60ml (p<0.0001) and 90ml (p<0.0001) respectively in pre-dose trough FEV1 at 2, 4 and 8 weeks of treatment from baseline in Glycopyrronium/Formoterol (25mcg/12mcg) group vs Glycopyrronium 50mcg group. (Fig 21)
Difference in average daily number of pMDI puffs of rescue medication consumed (ITT Population).
Difference in average daily number of pMDI puffs of rescue medication consumed at 2, 4, 8 and 12 week from baseline in Glycopyrronium/Formoterol (25mcg/12mcg) was -0.49 (p<0.0001), -0.74 (p<0.0001), -0.61 (p<0.0001) and -0.89 (p<0.0001) respectively while in Glycopyrronium 50mcg was -0.39 (p<0.0001), -0.72 (p<0.0001), -0.51 (p<0.0001) and -0.73 (p<0.0001) respectively. (Fig 22_)
Mean change in mMRC scale at 4, 8 and 12 weeks of treatment from baseline (ITT Population).
Mean change in mMRC scale at 4, 8 and 12 week from baseline in Glycopyrronium/Formoterol (25mcg/12mcg) group was -0.37 (p<0.0001), -0.57 (p<0.0001) and -0.85 (p<0.0001) respectively while in Glycopyrronium 50mcg group was -0.24 (p<0.0001), -0.44 (p<0.0001) and -0.65 (p<0.0001) respectively.(Fig 23)
Mean change in CAT score at 4, 8 and 12 weeks of treatment from baseline (ITT Population).
- Mean change in CAT score at 4, 8 and 12 week from baseline in Glycopyrronium/Formoterol (25mcg/12mcg) group was -3.49 (p<0.0001), -5.57 (p<0.0001) and -7.94 (p<0.0001) respectively and in Glycopyrronium 50mcg group was -2.32 (p<0.0001), -4.72 (p<0.0001) and -6.21 (p<0.0001) respectively.
- Minimum Clinically Important Difference (MCID) of 2 was observed in CAT score at 4, 8 and 12 week from baseline in both the treatment arms Glycopyrronium/Formoterol (25mcg/12mcg) and Glycopyrronium 50mcg. (Fig 24)
Mean change in COPD and Asthma Sleep Impact Scale (CASIS) score at 4, 8 and 12 weeks of treatment from baseline (ITT Population)
The percentage of nocturnal symptoms scores as per mean change in COPD and Asthma Sleep Impact Scale (CASIS) score improved consistently at 4, 8 and 12 weeks of treatment from baseline in both the Glycopyrronium/Formoterol (25mcg/12mcg) and Glycopyrronium (50mcg) groups. There was no difference between the 2 groups.
Conclusion
- Glycopyrronium/Formoterol (25mcg/12mcg) FDC was more effective than Glycopyrronium (50mcg) in improving lung functions and symptoms in patients with moderate to severe Chronic Obstructive Pulmonary Disease (COPD).
Safety of Glycopyrronium/ Formoterol Combination 19-21
In PINNACLE-1 and PINNACLE-2, 58.8% to 62.9% and 52.5% to 56.1% of patients, respectively, reported one or more AEs. Across both studies, the most common AEs included nasopharyngitis, cough, upper respiratory tract infection, sinusitis, and dyspnea, which occurred with a frequency similar to that with placebo MDI in each active treatment group.
The incidence of cardiovascular events of special interest was low and similar across treatment groups in both studies.
Few deaths were observed, and all were considered not related to the study drug. No important trends were observed in clinical laboratory results, vital signs, or electrocardiograms.
The incidence of AEs over 52 weeks, and the proportion considered related to treatment as judged by the investigator, was generally similar across treatment groups. The majority of AEs were mild or moderate in intensity and did not result in early discontinuation.
A subgroup of patients (n=585) in PINNACLE-2 were subjected to 24-h Holter monitoring study to further investigate the potential of cardiovascular events after treatment with GFF. The primary endpoint in the study was change from baseline in 24-h mean heart rate at week 4. It was reported that the changes from baseline in 24-h mean heart rate were similar across the treatment groups and the differences were not clinically relevant. No major changes in other important clinical parameters such as changes from baseline in day-time and night-time mean heart rates, frequency of isolated ventricular and supraventricular events, atrial fibrillation and mean 24-h heart rate were observed in the study. It was therefore evident that treatment with GFF was not associated with an increase in the risk of cardiovascular events
Safety of Glycopyrronium/Formoterol in Indian Patients 22
In the study in the Indian population, 35 adverse events were reported. The number of subjects with at least one adverse event were 32 (8.99%). One subject (0.28%) discontinued from the study due to adverse event. Most commonly reported adverse events were of the gastrointestinal system [dry throat 4 (1.12%)] and infections [upper respiratory tract infection 4 (1.12%)]. (Table 4)
No clinically significant changes were observed in vital signs and on physical examination.
The serum potassium values at weeks 4 and 12 post-treatment did not change significantly from baseline. No adverse events due to changes in serum potassium levels were reported in the study.
No adverse events related to variations in ECG were reported.
|
System Organ Class/ |
Glycopyrronium/ |
Glycopyrronium |
Overall |
|
Blood and lymphatic system disorders |
1(0.56%) |
0(0.00%) |
1(0.28%) |
|
Hypochromic anaemia |
1(0.56%) |
0(0.00%) |
1(0.28%) |
|
Gastrointestinal disorders |
4(2.25%) |
9(5.06%) |
13(3.65%) |
|
Abdominal pain |
0(0.00%) |
1(0.56%) |
1(0.28%) |
|
Diarrhoea |
0(0.00%) |
1(0.56%) |
1(0.28%) |
|
Dry mouth |
0(0.00%) |
2(1.12%) |
2(0.56%) |
|
Dry throat |
1(0.56%) |
3(1.69%) |
4(1.12%) |
|
Gastritis |
2(1.12%) |
1(0.56%) |
3(0.84%) |
|
Gastro-oesophageal reflux disease |
1(0.56%) |
0(0.00%) |
1(0.28%) |
|
Vomiting |
0(0.00%) |
1(0.56%) |
1(0.28%) |
|
General disorders and administration site conditions |
0(0.00%) |
1(0.56%) |
1(0.28%) |
|
Pyrexia |
0(0.00%) |
1(0.56%) |
1(0.28%) |
|
Nasopharyngitis |
1(0.56%) |
1(0.56%) |
2(0.56%) |
|
Pneumonia |
1(0.56%) |
0(0.00%) |
1(0.28%) |
|
Rhinitis |
0(0.00%) |
1(0.56%) |
1(0.28%) |
|
Upper respiratory tract infection |
3(1.69%) |
1(0.56%) |
4(1.12%) |
|
Musculoskeletal and connective tissue disorders |
2(1.12%) |
0(0.00%) |
2(0.56%) |
|
Arthralgia |
2(1.12%) |
0(0.00%) |
2(0.56%) |
|
Nervous system disorders |
2(1.12%) |
0(0.00%) |
2(0.56%) |
|
Headache |
2(1.12%) |
0(0.00%) |
2(0.56%) |
|
Renal and urinary disorders |
1(0.56%) |
1(0.56%) |
2(0.56%) |
|
Renal impairment |
1(0.56%) |
1(0.56%) |
2(0.56%) |
|
Respiratory, thoracic and mediastinal disorders |
1(0.56%) |
0(0.00%) |
1(0.28%) |
|
Chronic obstructive pulmonary disease |
1(0.56%) |
0(0.00%) |
1(0.28%) |
|
Skin and subcutaneous tissue disorders |
1(0.56%) |
0(0.00%) |
1(0.28%) |
|
Pruritus |
1(0.56%) |
0(0.00%) |
1(0.28%) |
|
Vascular disorders |
0(0.00%) |
1(0.56%) |
1(0.28%) |
|
Hypertension |
0(0.00%) |
1(0.56%) |
1(0.28%) |
Indications, Dosage and Administration
GLYCOHALE-F Rotacaps are indicated as a maintenance bronchodilator treatment to relieve symptoms in patients with chronic obstructive pulmonary disease (COPD).
The recommended dose is the inhalation of content of one capsule of GLYCOHALE-F Rotacaps twice daily using the Cipla Rotahaler or Revolizer device.
GLYCOHALE-F Rotacaps must not be swallowed.
GLYCOHALE-F Rotacaps is recommended to be administered, at the same time of the day each day. If a dose is missed, the next dose should be taken as soon as possible. Patients should be instructed not to take more than the prescribed doses in a day.
Warnings and Precautions
Serious Asthma-Related Events- Hospitalizations, Intubations, Death
• The safety and efficacy of GLYCOHALE-F Rotacaps in patients with asthma have not been established. GLYCOHALE-F Rotacaps is not indicated for treatment of asthma.
• Use of LABA as monotherapy [without inhaled corticosteroids (ICS)] for asthma is associated with an increased risk of asthma-related death. Available data from controlled clinical trials also suggest that use of LABA as monotherapy increases the risk of asthma-related hospitalization in pediatric and adolescent patients. These findings are considered a class effect of LABA monotherapy. When LABA are used in fixed-dose combination with ICS, data from large clinical trials do not show a significant increase in the risk of serious asthma-related events (hospitalizations, intubations, death) compared with ICS alone.
• No trial adequate to determine whether the rate of asthma-related deaths is increased in patients treated with Glycopyrronium/Formoterol has been conducted.
• Available data do not suggest an increased risk of death with use of LABA in patients with COPD.
Deterioration of Disease and Acute Episodes
• GLYCOHALE-F Rotacaps should not be initiated in patients during acutely deteriorating or potentially life-threatening episodes of COPD. Glycopyrronium/Formoterol has not been studied in subjects with acutely deteriorating COPD. The initiation of GLYCOHALE-F Rotacaps in this setting is not appropriate.
• GLYCOHALE-F Rotacaps should not be used for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm. Glycopyrronium/Formoterol has not been studied in the relief of acute symptoms and extra doses should not be used for that purpose. Acute symptoms should be treated with an inhaled, short-acting beta2-agonist.
• When beginning GLYCOHALE-F Rotacaps, patients who have been taking inhaled, short-acting beta2-agonists on a regular basis (e.g., four times a day) should be instructed to discontinue the regular use of these medicines and use them only for symptomatic relief of acute respiratory symptoms. When prescribing GLYCOHALE-F Rotacaps, the healthcare provider should also prescribe an inhaled, short-acting beta2-agonist and instruct the patient on how it should be used. Increasing inhaled beta2-agonist use is a signal of deteriorating disease for which prompt medical attention is indicated.
• COPD may deteriorate acutely over a period of hours or chronically over several days or longer. If GLYCOHALE-F Rotacaps no longer controls symptoms of bronchoconstriction; the patient’s inhaled, short-acting beta2-agonist becomes less effective; or the patient needs more inhalation of a short-acting beta2-agonist than usual, these may be markers of deterioration of disease. In this setting, a re-evaluation of the patient and the COPD treatment regimen should be undertaken at once. Increasing the daily dose of GLYCOHALE-F Rotacaps beyond the recommended dose is not appropriate in this situation.
Excessive Use of GLYCOHALE-F Rotacaps and Use with other Long-Acting Beta2-Agonists
• As with other inhaled medicines containing beta2-agonists, GLYCOHALE-F Rotacaps should not be used more often than recommended, at higher doses than recommended, or in conjunction with other medications containing LABAs, as an overdose may result.
• Clinically significant cardiovascular effects and fatalities have been reported in association with excessive use of inhaled sympathomimetic medicines.
• Patients using GLYCOHALE-F Rotacaps should not use another medicine containing a LABA for any reason [see Drug Interaction].
Paradoxical Bronchospasm
• As with other inhaled medicines, Glycopyrronium/Formoterol can produce paradoxical bronchospasm that may be life-threatening. If paradoxical bronchospasm occurs following dosing with GLYCOHALE-F Rotacaps, it should be treated immediately with an inhaled, short-acting bronchodilator; GLYCOHALE-F Rotacaps should be discontinued immediately, and alternative therapy should be instituted.
Immediate Hypersensitivity Reactions
• Immediate hypersensitivity reactions have been reported after administration of Glycopyrronium/Formoterol. If signs suggesting allergic reactions occur, in particular, angioedema (including difficulties in breathing or swallowing, swelling of the tongue, lips, and face), urticaria, or skin rash, GLYCOHALE-F Rotacaps should be discontinued immediately and alternative therapy should be instituted.
• GLYCOHALE-F Rotacaps should be used with caution in patients with severe hypersensitivity to milk proteins.
Cardiovascular Effects
• Formoterol fumarate, like other beta2-agonists, can produce a clinically significant cardiovascular effect in some patients as measured by increases in pulse rate, blood pressure, and/or symptoms [see Clinical Pharmacology]. Although such effects are uncommon after administration of Glycopyrronium/Formoterol at recommended doses, if they occur, GLYCOHALE-F Rotacaps may need to be discontinued.
• In addition, beta-agonists have been reported to produce ECG changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression. The clinical significance of these findings is unknown.
Therefore GLYCOHALE-F Rotacaps should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias and hypertension.
Worsening of Narrow-Angle Glaucoma
• GLYCOHALE-F Rotacaps should be used with caution in patients with narrow-angle glaucoma.
• Prescribers and patients should be alert for signs and symptoms of acute narrow-angle glaucoma (e.g., eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion and corneal edema).
• Instruct patients to consult a physician immediately should any of these signs or symptoms develop.
Worsening of Urinary Retention
• GLYCOHALE-F Rotacaps should be used with caution in patients with urinary retention. Prescribers and patients should be alert for signs and symptoms of urinary retention (e.g., difficulty passing urine, painful urination), especially in patients with prostatic hyperplasia or bladder-neck obstruction. Instruct patients to consult a physician immediately should any of these signs or symptoms develop.
Hypokalemia and Hyperglycemia
• Beta2-agonist medications may produce significant hypokalemia in some patients, possibly through intracellular shunting, which has the potential to produce adverse cardiovascular effects [see Clinical Pharmacology].
• The decrease in serum potassium is usually transient, not requiring supplementation. Beta2-agonist medicines may produce transient hyperglycemia in some patients.
• In two clinical trials of 24-weeks and a 28-week safety extension study evaluating glycopyrronium/ formoterol in subjects with COPD, there was no evidence of a treatment effect on serum glucose or potassium.
Coexisting Conditions
Glycopyrronium/ formoterol like all medications containing sympathomimetic amines, should be used with caution in patients with convulsive disorders or thyrotoxicosis and in those who are unusually responsive to sympathomimetic amines. Doses of the related beta2-agonist albuterol, when administered intravenously, have been reported to aggravate pre-existing diabetes mellitus and ketoacidosis.
Others
• GLYCOHALE-F Rotacaps contains lactose, which contains trace levels of milk proteins. Allergic reactions to products containing milk proteins may occur in patients with severe milk protein allergy.
Place of GLYCOHALE-F in Therapy
The GOLD guidelines recommend an individualized assessment of symptoms and future risk of exacerbations as the basis for the management strategy in patients with stable COPD. Regular administration of inhaled bronchodilators is central to symptom management. Use of a combination of bronchodilators with different mechanisms may be associated with better bronchodilation and reduced risk of adverse events (AE) s compared with increasing the doses of a single bronchodilator. Combination treatment with a LAMA/LABA is associated with increase in FEV1, reduction in symptoms and exacerbations compared with monotherapy.
The combination of the LAMA/LABA -glycopyrronium/formoterol has been shown to be safe and efficacious in the management of stable patients with moderate to severe COPD. The efficacy of glycopyrronium/formoterol as a pMDI formulation was established in the phase III PINNACLE programme in patients with moderate to very severe COPD. Glycopyrronium/formoterol was associated with significantly greater improvements from baseline than placebo or glycopyrronium or formoterol monotherapy in morning predose FEV1 at 24 weeks. The combination showed better improvement in morning predose FEV1 as compared to tiotropium at 52 weeks.
Glycopyrronium/formoterol was also associated with improvements in terms of peak change from baseline in FEV1 within 2 h postdose relative to placebo, monotherapies and tiotropium over 24 (PINNACLE-1, -2 and/or -4) or 52 (PINNACLE-3) weeks.
The combination improved dyspnoea to a greater extent than placebo and glycopyrronium monotherapy over 24 and 52 weeks (PINNACLE-1, -2, -3 and -4) ; significant differences in the SAC-TDI focal scores were also demonstrated relative to formoterol over 52 weeks (PINNACLE-3).
Glycopyrronium/formoterol significantly improved HR-QoL relative to placebo and glycopyrronium monotherapy in PINNACLE-1 and -4, both at and over 24 weeks, and relative to glycopyrronium monotherapy over 52 weeks in PINNACLE-3.
In addition, the risk of moderate/severe COPD exacerbations and the risk of treatment failure was reduced with glycopyrronium/formoterol treatment relative to the monotherapies and placebo over 24 weeks.
In Indian patients, the combination as a DPI resulted in improvement in lung function, reduction in use of rescue medication, improvement in breathlessness as measured by mMRC score as well as improvement in quality of life (as measured by CAT score)
Glycopyrronium/formoterol was generally well tolerated in patients with moderate to very severe COPD and had a similar AE profile to that of monotherapies, placebo and tiotropium. Glycopyrronium/formoterol was not associated with clinically meaningful effects on cardiovascular parameters. Over 52 weeks in PINNACLE-3, the incidence of major adverse cardiovascular events was low across the glycopyrronium/formoterol, glycopyrronium monotherapy, formoterol monotherapy and tiotropium groups, with no meaningful differences between the groups.
|
Glycopyrronium/formoterol a LAMA/LABA FDC as a DPI is an effective and generally well tolerated maintenance treatment for patients with moderate to very severe COPD. Current evidence suggests that it is a useful new addition that extends the available treatment options for the maintenance treatment of these patients.
|
Summary
- Optimization of bronchodilation is central to the management of patients with COPD.
- Robust and well-designed clinical trials have demonstrated the efficacy of Glycopyrronium/formoterol in terms of bronchodilation and symptom improvement in a wide range of disease severities.
- Glycopyrronium/ formoterol (25/12 mcg) is the optimum dose required to achieve clinically significant improvement in symptoms and quality of life of COPD patients.
- Twice daily dosing is more effective in reducing night time symptoms as compared to once daily regimen.
- In Indian patients with moderate-to-severe COPD, glycopyrronium/ formoterol (25/12 mcg) resulted in improvement in lung function, reduction in use of rescue medications and improvement in quality of life.
- The novel LAMA/LABA combination has a safety profile comparable to monotherapies and tiotropium.
References
- 2019 GLOBAL STRATEGY FOR PREVENTION, DIAGNOSIS AND MANAGEMENT OF COPD, Accessed from https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf,
- https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd)
- India State-Level Disease Burden Initiative CRD Collaborators, The burden of chronic respiratory diseases and their heterogeneity across the states of India: The Global Burden of Disease Study 1990–2016, Lancet Glob Health 2018; 6: e1363–74
- Al-Salama ZT, Frampton JE.Glycopyrronium/Formoterol: A Review in COPD. Drugs. 2019 Aug 29.
- Malay Sarkar, N Niranjan and PK Banyal, Mechanisms of hypoxemia, Lung India. 2017 Jan-Feb; 34(1): 47–60
- Rodrigo GJ, Price D, Anzueto A, Singh D et al LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2017 Mar 17;12:907-922
- Dejan Radovanovic, Marco Mantero, Francesco Giuseppe Sferrazza Papa, Vincenzo Valenti, Stefano Aliberti, Fabiano Di Marco & Pierachille Santus (2016): Formoterol fumarate + glycopyrrolate for the treatment of chronic obstructive pulmonary disease, Expert Review of Respiratory Medicine, DOI: 10.1080/17476348.2016.1227247
- Horita N, Goto A, Shibata Y, Ota E, Nakashima K, Nagai K, Kaneko T.Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2017 Feb 10;2:CD012066
- Penner, BM Formoterol dry-powder inhaler for the treatment of asthma and chronic obstructive pulmonary disease, Expert Opin. Pharmacother. (2007) 8(17):3069-3084
- FORADIL prescribing information, https://www.medicines.org.uk/emc/product/1030/smpc#PHARMACOKINETIC_PROPS
- Carter, NJ Inhaled Glycopyrronium Bromide: A Review of its Use in Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease, Drugs (2013) 73:741-753
- Buhl R, Banerji D.Profile of glycopyrronium for once-daily treatment of moderate-to-severe COPD.Int J Chron Obstruct Pulmon Dis. 2012;7:729-41
- Watz H, Mailänder C, May C, Baier M, Kirsten AM5.Fast onset of action of glycopyrronium compared with tiotropium in patients with moderate to severe COPD - A randomised, multicentre, crossover trial. Pulm Pharmacol Ther. 2017 Feb;42:13-20
- Seebri Prescribing information https://www.ema.europa.eu/en/documents/product-information/seebri-breezhaler-epar-product-information_en.pdf
- Aalbers R, Ayres J, Backer V, Decramer M, Lier PA, Magyar P, Malolepszy J, Ruffin R, Sybrecht GW.Formoterol in patients with chronic obstructive pulmonary disease: a randomized, controlled, 3-month trial.Eur Respir J. 2002 May;19(5):936-43.
- Bogdan MA, Aizawa H, Fukuchi Y, Mishima M, Nishimura M, Ichinose M. Efficacy and safety of inhaled formoterol 4.5 and 9 ?g twice daily in Japanese and European COPD patients: Phase III study results. BMC pulmonary medicine. 2011 Dec;11(1):51.
- Verkindre C, Fukuchi Y, Flémale A, Takeda A, Overend T, Prasad N, Dolker M. Sustained 24-h efficacy of NVA237, a once-daily long-acting muscarinic antagonist, in COPD patients. Respiratory medicine. 2010 Oct 1;104(10):1482-9.
- Arievich H, Overend T, Renard D, Gibbs M, Alagappan V, Looby M, Banerji D.A novel model-based approach for dose determination of glycopyrronium bromide in COPD. BMC Pulm Med. 2012 Dec 8;12:74.
- Martinez FJ, Rabe KF, Ferguson GT, Fabbri LM, Rennard S, Feldman GJ, Sethi S, Spangenthal S, Gottschlich GM, Rodriguez-Roisin R, Arora S, Siler TM, Siddiqui S, Darken P, Fischer T, Maes A, Golden M, Orevillo C, Reisner C. Efficacy and Safety of Glycopyrrolate/Formoterol Metered Dose Inhaler Formulated Using Co-Suspension Delivery Technology in Patients With COPD.Chest. 2017 Feb;151(2):340-357
- Rabe KF GFF MDI for the improvement of lung function in COPD - A look at the PINNACLE-1 and PINNACLE-2 data and beyond. Expert Rev Clin Pharmacol. 2017 Jul;10(7):685-698
- Hanania NA, Tashkin DP, Kerwin EM, Donohue JF, Denenberg M, O'Donnell DE, Quinn D, Siddiqui S, Orevillo C, Maes A, Reisner C.Long-term safety and efficacy of glycopyrrolate/formoterol metered dose inhaler using novel Co-Suspension™ Delivery Technology in patients with chronic obstructive pulmonary disease. Respir Med. 2017 May;126:105-115.
- Data on file