Epidemiology
Rapid expansion of services to prevent mother-to-child transmission of HIV has had a major health impact on reducing new HIV infections among children over the past 30 years. Antiretroviral medicines have averted an estimated 1.4 million HIV infections among children since 2000.1
Global Statistics
HIV/AIDS has had a profound impact on children around the world since the start of the epidemic. In 2014, there were 2.6 million children under the age of 15 years living with HIV globally and 2.3 million of them were in Sub-Saharan Africa.1
2014 Global HIV and AIDS estimates (Children <15 years)1,3
Children living with HIV : 2.6 million [2.4 million–2.8 million]
New HIV infections among children : 220,000 [190,000–260,000]
AIDS-related deaths : 150,000 [140,000–170,000]
Prevalence in India3
Total number of children living with HIV less than 15 years of age accounted for 6.54% of the total 21.17 lakh people living with HIV (PLHIV) in India.
References
- UNAIDS Report on MDG 6 Goal 2015. http://www.unaids.org/sites/default/files/media_asset/MDG6Report_en.pdf Accessed on 17th April 2016.
- UNAIDS report on 2015 World AIDS DAY http://www.unaids.org/sites/default/files/media_asset/20150901_FactSheet_2015_en.pdf Accessed on 17th April 2016.
- http://www.naco.gov.in/upload/2015%20MSLNS/HSS/India%20HIV%20Estimations%202015.pdf Accessed on 17th April 2016.
Transmission of HIV
The mother-to-child transmission (MTCT) of HIV refers to the transmission of HIV from an HIV-positive woman to her child during pregnancy, labour, childbirth or breastfeeding. It is by far the most common way in which about 90% of the children become infected with HIV.1,2
High mortality rates are seen in the first year of life among HIV-infected infants who are untreated, which makes early HIV testing, prompt return of results and rapid initiation of treatment essential.3 Without prophylactic treatment the possibility of HIV passing from mother-to-child is 15–45%1,4,5 during gestation and delivery and further 5–15% during breastfeeding.5
In June 2016, the WHO released new guidelines recommending lifelong antiretroviral treatment (ART) for all pregnant and breastfeeding women living with HIV even if they are identified late in pregnancy or postpartum to reduce the risk of MTCT (Option B+)1,2,6,7 along with infant prophylaxis with AZT (twice daily) and NVP (once daily) for the first 6 weeks of life, whether they are breastfed or formula fed. For further details please refer to Infant prophylaxis page 19.
References
1. http://www.avert.org/node/375/pdf Last accessed on 18th April 2016.
2. https://aidsinfo.nih.gov/education-materials/fact-sheets/20/50/prevention-of-mother-to-child-transmission-of-hiv Last accessed on 18th April 2016.
3. Consolidated guidelines on HIV testing services – WHO 2015.
4. http://www.wpro.who.int/hiv/topics/pmtct/about/en/ Last accessed 18th April 2016
5. Global guidance on criteria and processes for validation: elimination of mother-to-child transmission of hiv and syphilis.
6. http://www.emtct-iatt.org/wp-content/uploads/2015/05/IATT-Framework-May-2015.pdf Last accessed 18th April 2016.
7. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach WHO 2016, 2nd Edition.
Pathogenesis and Natural History of HIV-1 Infection in Children
Pathogenesis
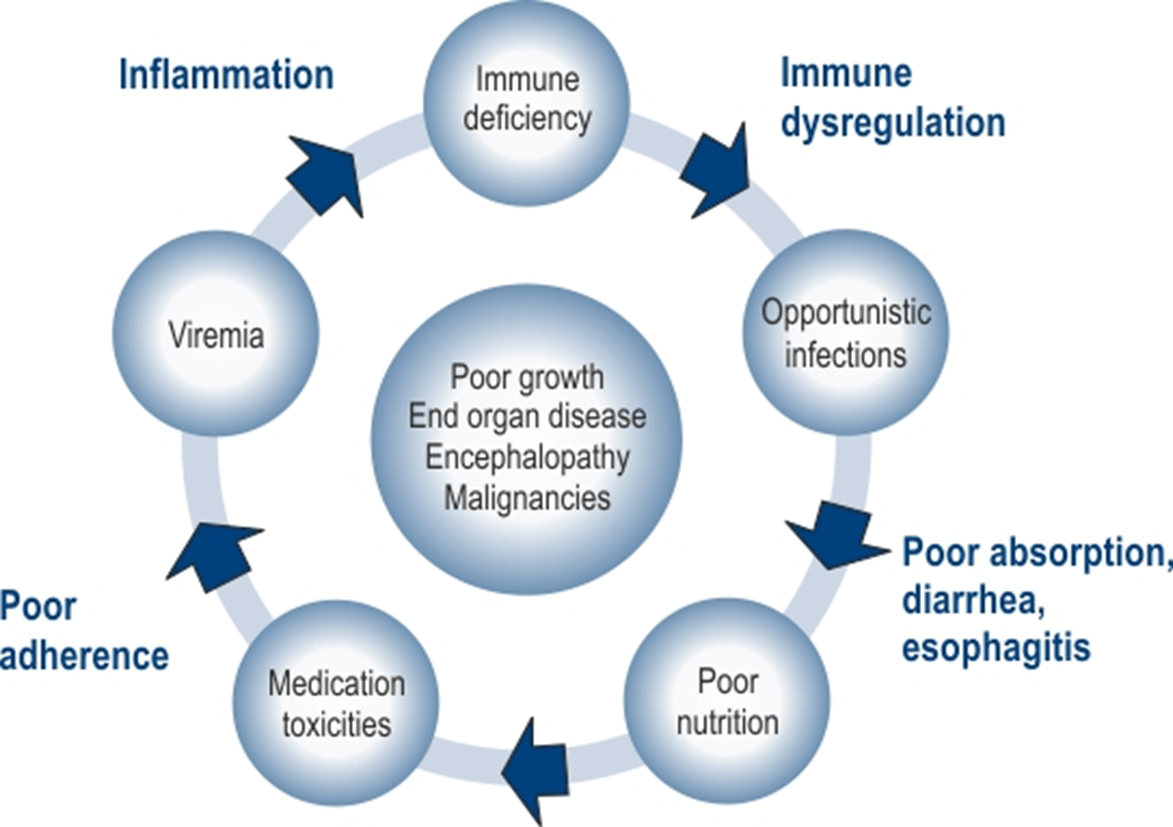
The pathogenesis of paediatric primary HIV-1 infection appears to differ significantly from adults in that it is characterized by much higher viral load levels, more rapid disease progression, and early central nervous system disease. Such different manifestations may be related in part to immaturity of the infant’s immune system, which results in altered host responses to HIV infection among infants and young children compared to adults. Historically, a high proportion of children (about 10-20%) experienced rapid progression of the disease and died of AIDS-related complications within the first 4 years of primary infection.1,2,3
Natural History of HIV Infection in Children2,3,4,5,6,7
The natural history of HIV disease in children differs from adults. Children who acquire HIV during pregnancy, birth or breastfeeding can be divided into two groups: rapid progressors and slow progressors. The first group constitutes of infants who have high levels of detectable virus at birth. The disease progresses rapidly, and these children manifest severe clinical symptoms and immunologic abnormalities within the first years of life, including HIV encephalopathy, often the presenting condition that indicates AIDS. About 15–20% of infants develop this early severe form of the disease and are called as rapid progressors. The remaining 80–85% have a form of disease that progresses more slowly and is more similar to that seen in adults.2 The factors underlying the difference between the two groups are unclear but may relate to the timing of HIV-1 infection (during gestation versus parturition), the stage of maternal HIV-1 disease during pregnancy and delivery (maternal viraemia), the neonates’ viral load, the virulence of the infecting virus, and host-related factors.
Signs and Symptoms of Paediatric HIV infection:1,8
- Unusually frequent and severe occurrences of common childhood bacterial infections, such as otitis media, sinusitis, and pneumonia.
- Recurrent fungal infections, such as candidiasis (thrush), that do not respond to standard antifungal agents; suggests lymphocytic dysfunction.
- Recurrent or unusually severe viral infections, such as recurrent or disseminated herpes simplex or zoster infection or cytomegalovirus (CMV) retinitis; seen with moderate-to-severe cellular immune deficiency.
- Growth failure
- Failure to thrive
- Wasting
- Failure to attain typical milestones; suggests a developmental delay. Such delays, particularly impairment in the development of expressive language, may indicate HIV encephalopathy.
- Behavioural abnormalities (in older children), such as loss of concentration and memory, may also indicate HIV encephalopathy.
References
1. Indian J Pediatr. 2006 Mar;73(3):201-4.Clinical profile and natural history of children with HIV infection.
2. Clin. Microbiol. Rev. 1996, 9(4):448. Pediatric Human Immunodeficiency Virus Infection Joseph B. Domachowske.
3. Curr HIV/AIDS Rep. 2006 February ; 3(1): 13–19. Immune pathogenesis of pediatric HIV-1 infection.
4. HIV care and Counselling 4th Edition.
5. Oxford Journal, British medical bulletin, vol 58,Issue 1pp89-108 Paediatric HIV infection: correlates of protective immunity and global perspectives in prevention and management.
6. N Engl J Med 1997; 336:1337-1342May 8, 1997DOI: 10.1056/NEJM199705083361901 Viral Load and Disease Progression in Infants Infected with Human Immunodeficiency Virus Type 1.
7. Mamtha Lala, Rashid Merchant; Principles of Perinatal and Pediatric HIV/AIDS; 12th Edition; India; Jaypee; 2012.
8. Pediatric HIV infection. January 2014. Medscape. Http://emedicine.medscape.com/article/965086-overview.
Diagnosis
It is important to diagnose HIV-exposed-infants as early as possible, so as to start antiretroviral therapy and prevent early morbidity and mortality.
Infection can be diagnosed only by virological testing in infants and children less than 18 months of age as maternal HIV antibodies remain in the infant’s bloodstream until 18 months of age. Virological testing is most commonly performed on dried blood spot specimens.1
Standard HIV serological assays such as RDTs and EIAs can reliably determine HIV status of children aged 18 months and older.1
WHO Recommendations1,2
- HIV virological testing should be done in all HIV-exposed infants at 4 to 6 weeks of age or at the earliest opportunity thereafter.
- Well HIV-exposed infants are recommended to undergo HIV serological testing at around 9 months of age.
- Infants who have reactive serological assays at 9 months of age should have a virological test to identify HIV-infected infants who need ART.
- HIV serological testing should be performed on children 18 months of age or older with suspected HIV infection or HIV exposure, according to the validated national testing algorithm used in adults.
- All infants with signs or symptoms suggestive of HIV infection should undergo HIV serological testing and if reactive, should be referred for virological testing.
HIV testing should be offered to all children and adolescents presenting with indicator conditions or signs and symptoms that suggest HIV, including oral candidiasis, failure to thrive, chronic cough and skin conditions or attending TB clinics and malnutrition services in all settings.
References
1. Consolidated guidelines on HIV testing services – WHO 2015.
2. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach WHO 2016, 2nd Edition.
Introduction to Antiretroviral Therapy (ART)
The prospects for using ART drugs for treating and preventing HIV infection effectively are growing rapidly, thus providing a chance for children infected with HIV to lead a normal life. However, ART in children has many challenges: use of appropriate formulations, regular need for modification of doses as the child grows, adherence issues, etc.
The goals of ART for HIV-infected children and adolescents include the following:
- Preventing and reducing HIV-related morbidity and mortality.
- Restoring and/or preserving immune function as reflected by CD4 cell measures.
- Maximally and durably suppressing viral replication.
- Preventing emergence of viral drug-resistance mutations.
- Minimizing drug-related toxicity.
- Maintaining normal physical growth and neurocognitive development.
- Improving quality of life.
- Reducing the risk of sexual transmission to discordant partners in adolescents who are sexually active.
- Reducing the risk of perinatal transmission in adolescent females who become pregnant.
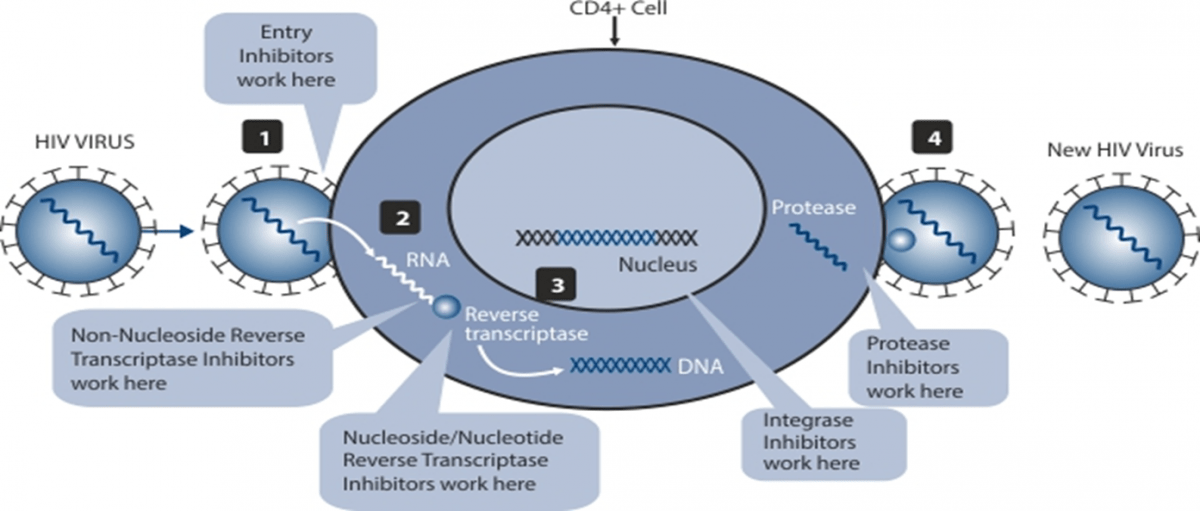
Currently available ART utilizes five major classes of Antiretroviral (ARV) drugs:
- Nucleoside/nucleotide Reverse Transcriptase Inhibitors (NRTIs) – Inhibit the enzyme reverse transcriptase and incorporate the nucleotide into the DNA chain, thus causing chain termination.
- Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) – Inhibit the enzyme reverse transcriptase at the site of enzyme action.
- Protease Inhibitors (PIs) – Inhibit the enzyme protease and prevents the cleavage of the long-chain proteins.
- Entry and Fusion Inhibitors – Inhibit the binding of the virus to the receptors on the surface of the cells.
- Integrase Inhibitors (INTIs) – Inhibit the enzyme integrase and prevent the intergration of the viral DNA into the cellular DNA.
Initiating ART in Children
Access to early infant diagnosis has improved the identification of infants living with HIV. Infants and young children have an exceptionally high risk of poor outcomes from HIV infection. Dependency on a caregiver, diagnosis and retaining children exposed to and living with HIV is a challenge, in particular with loss to follow-up cases.
However, the ART initiation rate among HIV-infected children in 2014 was 32%, which shows a considerable lag with that of the adults (41%).1
The recent 2016 WHO guidelines have recommended to initiate ART in all children with HIV, aligning the recommendations for children with that of adults and adolescents.
WHO 2016 Recommendations2
|
Adolescents (10–19 years old) |
Children (1 to <10 years old) |
Children (<1 year old) |
|
|
|
ART: What to Start
The delivery of affordable, child-friendly, and easy to store and administer paediatric ART is a key to the successful management of paediatric HIV disease. Simplified, less toxic and more convenient regimens as fixed-dose combinations are recommended for first-line ART.
The revised recommendations on when to start ART in all children and adolescents are elaborated in the table below:
WHO 2016 Guidelines2
|
|
Children aged less than 3 years |
Children aged 3 to 10 years |
Adolescents |
|
Preferred regimens |
ABCa or AZT + 3TC+ LPV/rb |
ABC +3TC+EFV |
TDF+3TC(or FTC)+EFVc |
|
Alternative regimens d |
ABCa or AZT + 3TC + NVP |
ABC + 3TC + NVP AZT + 3TC + EFV AZT + 3TC + NVP TDF + 3TC (or FTC) + EFV TDF + 3TC (or FTC) + NVP |
TDF (or ABC) + 3TC (or FTC) + DTG TDF (or ABC) + 3TC (or FTC) + EFV400 ABC + 3TC + EFV ABC + 3TC + NVP AZT + 3TC + EFV AZT + 3TC + NVP TDF + 3TC (or FTC) + NVP |
|
Special circumstances e,g |
ABCa or AZT + 3TC + RALf |
|
Regimens containing boosted PIs |
a Based on the general principle of using non-thymidine analogues in first-line regimens and thymidine analogues in second-line regimens, ABC should be considered as the preferred NRTI whenever possible. Availability and cost should be carefully considered.
b As recommended by the US FDA, using LPV/r oral liquid should be avoided in premature babies (born 1 month or more before the expected date of delivery) until 14 days after their due date or in full-term babies younger than 14 days of age. Dosing for children younger than 6 weeks should be calculated based on body surface area. Restrictions also apply to LPV/r pellets, where administration challenges extend to infants up to 3 months of age. Additional information regarding optimal administration of this formulation will be provided as more data become available.
c To date, there is limited experience with the use of low-dose EFV and DTG in adolescents. While no age or weight restrictions apply to the use of EFV 400 mg/day, which can be used starting from a weight of 20 kg, the use of DTGis approved only for adolescents who are older than 12 years and weigh more than 40 kg. In addition, safety and pharmacokinetic data on TB coinfection and pregnancy are still pending.
d Challenges may arise when treatment is started in the first two weeks of life following early diagnosis at or around birth, particularly in case of prematurity or low birth weight. In these situations, an NVP-based regimen containing AZT and 3TC should be started, and NVP should be substituted with LPV/r at the earliest opportunity, preferably at two weeks when LPV/r syrup can be administered. In settings where LPV/r syrup is not available and LPV/r pellets are the only formulation available, administration of NVP should continue until 3 months with close clinical monitoring for those children considered at high risk for carrying NNRTI resistance (i.e. prolonged NVP-based postnatal prophylaxis or documented NNRTI failure in the mother). Only applicable for children aged less than 3 years.
e Special circumstances may include situations where preferred or alternative regimens may not be available or suitable because of significant toxicities, anticipated drug–drug interactions, drug procurement and supply management issues or for other reasons.
f RAL is approved for use in infants and children from the age of 4 weeks, but there is very limited evidence to inform the use of raltegravir (RAL) as a first-line drug in infants and young children (336). The use of this INSTI could be considered where available in instances of poor tolerability or administration challenges with LPV/r, particularly in settings where as a result of rapid expansion of maternal treatment, infants and children are at very high risk of carrying an NNRTI resistance virus. Use of RAL should however consider the challenges of existing granule formulation, despite being suitable for use in infants 4 weeks and older, as reconstitution in water is required before administration. While dispersion of RAL chewable tablets is considered to be a potential alternative, additional information regarding the appropriateness of this approach will be provided as more data become available.
g Using d4T as an option in first-line treatment should be discontinued.
|
|
0-2 Weeks → |
2 Weeks-3 months → |
3-36 months |
|
Preferred |
AZT+3TC+NVP |
ABC or AZT + 3TC + LPV/r syrup |
ABC or AZT+3TC+LPV/r pellets |
|
Alternative |
AZT+3TC+NVP |
ABC or AZT+3TC+LPV/r pellets |
|
|
Special circimstances |
AZT+3TC + NVP |
ABC or AZT+3TC+RAL |
|
References
1. UNAIDS Report on MDG 6 Goal 2015. http://www.unaids.org/en/resources/campaigns/HowAIDSchangedeverything/ Factsheet Accessed on 1st April 2016.
2. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach WHO 2016, 2nd Edition.
Monitoring1
- More than 80% of children identified as living with HIV would be eligible for ART, based on the 2013 recommendations. Therefore, laboratory monitoring will need to be strengthened to monitor treatment efficacy and identify treatment failures among children.
- Growth monitoring in the routine clinical assessment of children living with HIV suggests that loss of weight or poor weight gain are potential signs of treatment failure.
- Careful clinical monitoring is essential to assess the risk of treatment failure among children on ART
- With limited drug options available for lifelong treatment, HIV-infected infants and children for whom CD4-based criteria are particularly poor, preferential viral load testing should be offered.
- CD4 monitoring will also be important for tracking the treatment response, in cases where viral load monitoring capacity is limited or unavailable.
References
1. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach WHO 2016, 2nd Edition.
Management of Treatment Failure1
WHO Definition of Treatment Failure
Clinical Failure
- New or recurrent clinical event indicating advanced or severe immunodeficiency (WHO clinical stages 3 and 4 clinical condition with the exception of TB) after 6 months of effective treatment.
- The condition must be differentiated from immune reconstitution inflammatory syndrome occurring after initiating ART.
Immunological Failure
- Children younger than 5 years of age
- Persistent CD4 levels below 200 cells/mm3.
- Older than 5 years of age
- Persistent CD4 levels below 100 cells/mm3.
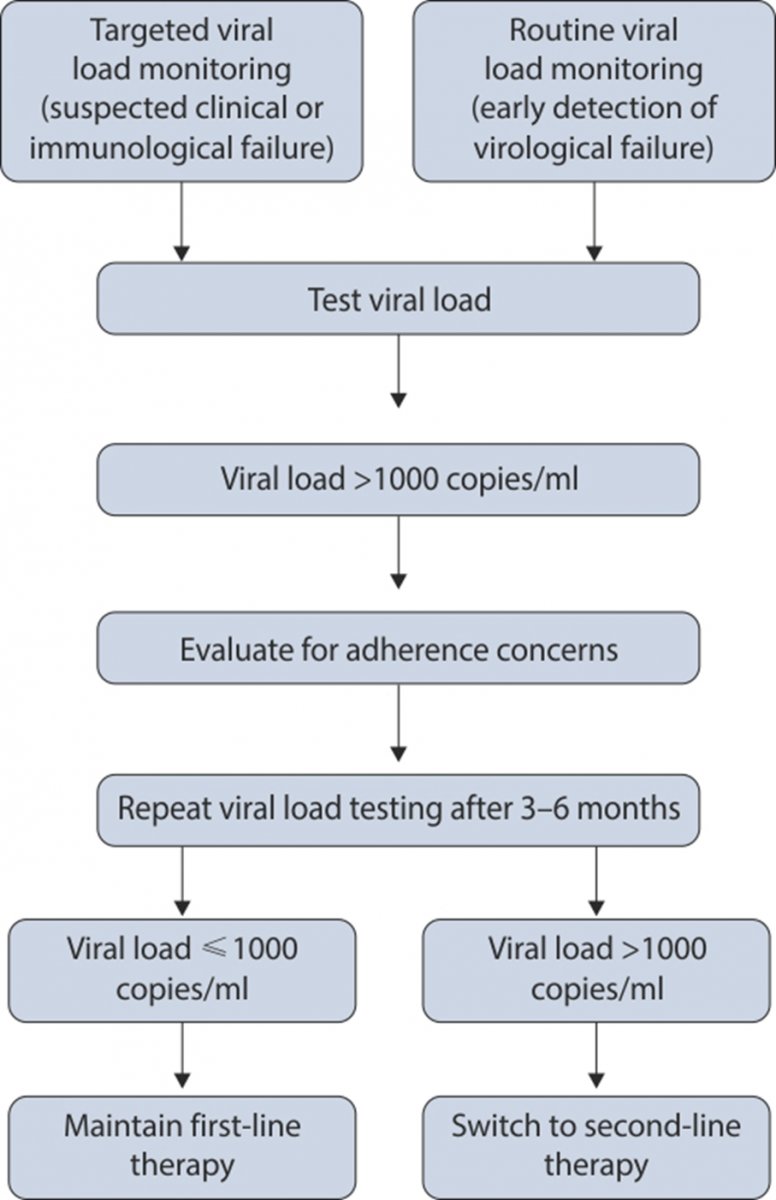
Virological Failure
- Plasma viral load above 1,000 copies/ml based on two consecutive viral load measurements after 3 months, with adherence support.
- An individual must be taking ART for at least 6 months before it can be determined that a regimen has failed.
switch ART regimen in adults, adolescents and children1
ART Options after Treatment Failure
|
|
Children (including adolescents) |
First-line ART regimen |
Second-line ART regimen |
|
LPV/r-based first-line regimen |
Younger than 3 years |
ABC+3TC+LPV/r |
AZT or ABC+3TC+RALa |
|
AZT+3TC+ LPV/r |
|||
|
3 Years and older |
ABC+3TC+LPV/r |
AZT+3TC+EPV or RAL |
|
|
AZT+3TC+ LPV/r |
ABC or TDFb +3TC+EFV or RAL |
||
|
NNRTI based first-line regimen |
All ages |
ABC+3TC+EFV(or NVP) |
AZT+3TC+ATV/r or LPV/rc |
|
TDFb+3TC(or FTC)+EFV (or NVP) |
|||
|
AZT+3TC+EFV (or NVP) |
ABC or TDV+3TCc (or FTC)+ATV/r or LPV/rc |
a If RAL is not available, no change is recommended unless in the case of advanced clinical disease progression or lack of adherence specifically due to poor palatability of LPV/r. In this case, switching to a second-line NVP-based regimen should be considered. Based on approval of the use of EFV in children less than 3 years, an EFV-based regimen could be considered as an alternative. However, more data are needed to inform how best to use EFV in this population.
b TDF may be given only to children older than 2 years.
c ATV/r can be used as an alternative to LPV/r in children older than 3 months. However, the limited availability of suitable formulations for children younger than 6 years, the lack of an FDC and the need for separate administration of the RTV booster should be considered when choosing this regimen.
Reference
1. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach WHO 2016, 2nd Edition.
Management of Co-infections in Children
HIV/TB Co-Infection
Tuberculosis is one of the most common opportunistic infections affecting children living with HIV. Co-treatment in children under three years of age is challenging due to drug interactions between rifampicin and lopinavir/ritonavir or nevirapine.
Managing HIV/TB Co-infection1
ART in HIV/TB Co-infected Children: When to start
- ART should be started in all TB patients, including those with drug-resistant TB, irrespective of the CD4 count.
- Anti-TB treatment should be initiated first, followed by ART as soon as possible within the first 8 weeks of treatment.
- The HIV-positive TB patients with profound immunosuppression (such as CD4 counts <50 cells/mm3) should receive ART immediately within the first 2 weeks of initiating TB treatment.
- ART should be started in any child with active TB disease as soon as possible and within 8 weeks following the initiation of anti-TB treatment, irrespective of the CD4 count and clinical stage.
- Efavirenz is also the preferred NNRTI for people with HIV and TB.
- The WHO recommends ART for all patients with HIV and drug-resistant TB, requiring second-line anti-TB drugs irrespective of CD4 cell count, as early as possible (within the first 8 weeks) following initiation of anti-TB treatment.
|
Recommended regimens for children and adolescents initiating ART while on TB treatmenta,b |
||
|
Younger than 3 years |
Triple NRTI (AZT+3TC+ABC)c |
|
|
3 years and older |
Two NRTIs+EFV or Triple NRTI (AZT+3TC+ABC)c |
|
|
Recommended regimen for children and infants initiating TB treatment while receiving ARTa |
||
|
Child on standard NNRTI-based regimen (two NRTIs + EFV or NVP) |
Younger than 3 years |
Continue NVP, ensuring that dose is 200 mg/m2 or Triple NRTI (AZT+3TC+ABC)c |
|
3 years and older |
If the child is receiving EFV, continue the same regimen If the child is receiving NVP, substitute with EFV or Triple NRTI (AZT+3TC+ABC)c |
|
|
Recommended regimen for children and infants initiating TB treatment while receiving ARTa |
||
|
Child on standard PI-based regimen (two NRTIs + LPV/r) |
Younger than 3 years |
Triple NRTI (AZT+3TC+ABC)c or Continue LPV/r; adding RTV to achieve the full therapeutic dosed |
|
3 years and older |
If the child has no history of failure of an NNRTI-based regimen: Substitute with EFVe or Triple NRTI (AZT+3TC+ABC)c or Continue LPV/r; adding RTV to achieve the full therapeutic dosed If the child has a history of failure of an NNRTI-based regimen: Triple NRTI (AZT+3TC+ABC)c or Continue LPV/r adding RTV to achieve the full therapeutic dosed Consider consultation with experts for constructing a second-line regimen |
|
|
a Ensure optimal dosing of rifampicin based on dosing guidelines b Substitute ARV drugs based on an age-appropriate ART regimen in line with nationally recommended first-line ART. c Triple NRTI is only recommended for the duration of TB treatment; an age-appropriate PI- or NNRTI-based regimen should be restarted when rifampicin-based therapy ends. Based on the findings from the ARROW trial, this regimen should be considered as the preferred option for children younger than three years who are receiving a LPV/r-based regimen when starting TB treatment. The United States Food and Drug Administration approval for the use of EFV in children 3 months to 3 years old weighing more than 3.5 kg offers a potential alternative to the triple NRTI approach. An EFV-based regimen in children under 3 years is still not recommended as pharmacokinetics data are needed to ensure that the co-administration of rifampicin does not decrease drug levels below the therapeutic level. Triple NRTI should also be considered as the preferred regimen for children older than 3 years with a history of failure on a NNRTI-based regimen. d Increase RTV until it reaches the same dose as LPV in mg, in a ratio of 1:1. e Substitution with EFV should be considered as the preferred option, and EFV could be maintained after TB treatment ends to enable simplification and harmonization with the ARV drug regimens used for older children. |
||
Recommendation for Isoniazid Preventive Therapy (IPT)1
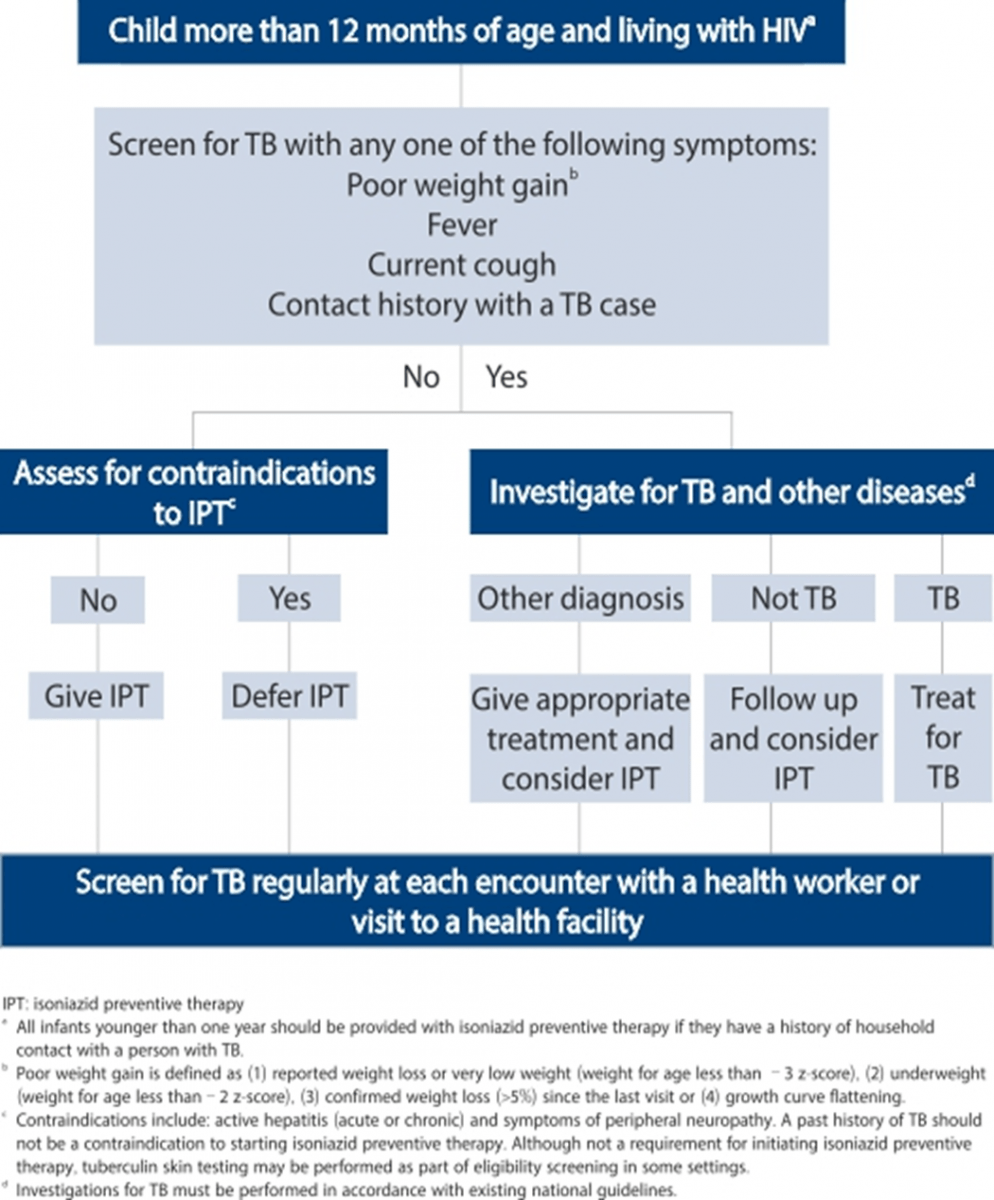
- Children living with HIV who do not have poor weight gain, fever or current cough are unlikely to have active TB.
- Children living with HIV who are more than 12 months of age and who are unlikely to have active TB on symptom-based screening and have no contact with a TB case, should receive 6 months of IPT (10 mg/kg/day) as part of a comprehensive package of HIV prevention and care services.
- In children living with HIV who are less than 12 months of age, only those who have contact with a TB case and who are evaluated for TB (using investigations) should receive 6 months of IPT if the evaluation shows no TB disease.
- All children living with HIV, after successful completion of treatment for TB disease, should receive isoniazid for an additional 6 months.
HIV/Hepatitis Co-infection
Viral hepatitis progresses faster and causes more liver-related complications among individuals with HIV than among those who do not have HIV.3 The prevalence of chronic viral hepatitis in HIV-infected children is not well characterized however, perinatal transmission was reported as the most common mode of HBV and HCV transmission in children.4,5
Children with HIV/HBV Co-infection
Small cohort studies in countries in which both HIV and HBV were endemic, reported a prevalence rate of chronic HBV among children with HIV to be between 1% and 49%.4 In addition to HBeAg positivity and HBV genotype, high-maternal viral load (HBV DNA) significantly increased transplacental transmission HBV.4
ART in HIV/HBV Co-infected Children: When and what to start
Initiate ART in people with HBV co-infection with severe liver disease and in all children younger than 5 years of age living with HIV regardless of CD4 cell count. Including TDF in initial ART regimens for children with HBV co-infection offers the potential advantage of reducing the selection of HIV resistance to 3TC that may compromise future options for HBV treatment.
For individuals co-infected with HIV and HBV whose first-line regimen contained TDF + 3TC (or FTC), these NRTIs should be continued in the second-line regimen for the anti-HBV activity and to reduce the risk of hepatic flares, regardless of the selected second-line regimen, which should be AZT + TDF + 3TC (or FTC) + Boosted PIs.1
Children with HIV/HCV Co-infection
Few studies that have focused on vertical transmission of HIV/HCV co-infection found that HCV transmission rates from HIV/HCV-co-infected mothers ranged from 5·4% to 23% and were substantially higher than those from HCV-only infected mothers.5
ART in HIV/HCV Co-infected Children: When and what to start
Initiating ART in people co-infected with HCV should follow the same general principles as in people with HIV mono-infection.
People co-infected with HIV and HCV receiving ART and HCV drugs require close monitoring because of potential drug interactions and increased risk for drug toxicity between HCV drugs and ARV drugs. Also, people receiving AZT may need to switch to TDF.1
|
First-line ART |
Preferred first-line regimens |
Alternative first-line regimensa,b |
|
Adults and adolescents (including pregnant and breastfeeding women and adults with TB co-infection and HBV co-infection) |
TDF+3TC (or FTC) + EFV as a fixed-dose combination
|
AZT+3TC+EFV AZT+3TC+NVP TDF+3TC (or FTC)+NVP |
|
Children ≥3 years |
ABC+3TC+EFV |
ABC+3TC+NVP AZT+3TC+EFV AZT+3TC+NVP TDF+3TC (or FTC)+EFV TDF+3TC (or FTC)+NVP |
|
Children < 3 years |
ABC (or AZT)+3TC+ LPV/r |
ABC+3TC+NVP AZT+3TC+NVP |
|
a ABC or boosted PIs (ATV/r, DRV/r , LPV/r) can be used in special circumstances. b Countries should discontinue d4T use in first-line regimens because of its well-recognized metabolic toxicities. For adults, using d4T as an option in first-line treatment should be discontinued and restricted to special cases in which other ARV drugs cannot be used and to the shortest time possible, with close monitoring. For children, d4T use should be restricted to situations in which there is suspected or confirmed toxicity to AZT and lack of access to ABC or TDF. The duration of therapy with this drug should be limited to the shortest time possible. |
||
References
1. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach WHO 2016, 2nd Edition.
2. WHO Guidance for national tuberculosis programmes on the management of
tuberculosis in children - 2014
2nd Edition.
3. http://www.cdc.gov/hiv/pdf/library_factsheets_hiv_and_viral_hepatitis.pdf Last accessed May 2016.
4. Expert Rev Anti Infect Ther. 2013; 11(3):251–63.
5. Lancet Infect Dis 2006; 6: 83–90.
6. Guidelines for the prevention, care and treatment of persons with chronic Hepatitis B infection WHO 2015.
7. Guidelines for the screening, care and treatment of persons with hepatitis C infection WHO 2014.
Use of ART for Infant Prophylaxis1
Infant prophylaxis has shown to be particularly important for PMTCT when the mother has received limited or no antepartum ARV drugs and when viral suppression has not yet been achieved. Infant prophylaxis is also important when a breastfeeding mother interrupts ART during breastfeeding, as this places her infant at an increased risk of postnatal transmission. In such situations, providing daily infant NVP during the period of maternal ART interruption should be considered, and this could be stopped 6 weeks after maternal ART is restarted (or 1 week after breastfeeding ends, whichever comes first).
Infant prophylaxis should begin at birth or when HIV exposure is recognized postpartum. Infants born to mothers with HIV who are at high risk of acquiring HIV-1 should receive dual prophylaxis with daily AZT and NVP for the first 6 weeks of life, whether they are breastfed or formula-fed.2
Infants of mothers who are receiving ART and are breastfeeding should receive 6 weeks of infant prophylaxis with daily NVP. Breastfed infants who are at high risk of acquiring HIV-1 including those first identified as exposed to HIV during the postpartum period, should continue infant prophylaxis for an additional 6 weeks (total of 12 weeks of infant prophylaxis) using either AZT and NVP or NVP alone.2
If infants are receiving replacement feeding, they should be given 4–6 weeks of infant prophylaxis with daily NVP (or twice-daily AZT).
If toxicity from infant NVP occurs and it requires discontinuation of the drug or if infant NVP is not available, infant 3TC can be substituted.
|
Infant age |
Dosing of NVP |
Dosing of AZT |
|
Birth to 6 weeks |
|
|
|
Birth weight 200-2499ga |
10 mg once daily |
10 mg twice daily (1 ml of syrup twice daily) |
|
Birth weight ≥ 2500 g |
15 mg once daily (1.5 ml of syrup once daily) |
15 mg twice daily (1.5 ml of syrup twice daily) |
|
>6 weeks to 12 weeks |
|
|
|
|
20 mg once daily (2 ml of syrup once daily or half a 50 mg tablet once daily) |
No dose established for prophylaxis; use treatment dose 60 mg twice daily 6 ml of syrup twice daily or a 60 mg tablet twice daily |
a For infants weighing <2000 g and older than 35 weeks of gestational age, the suggested doses are: NVP 2 mg/kg per dose once daily and AZT 4 mg/kg per dose twice daily. Premature infants younger than 35 weeks of gestational age should be dosed using expert guidance.
Reference
1. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach WHO 2016, 2nd Edition.
Adherence to ART in Children1
- Adherence among children is a special challenge. Some of the issues with ARVs for children are the limited available options of paediatric formulations, poor palatability of liquid formulations, high pill or liquid volume burden, large pill size, frequent dosing requirements, dietary restrictions, loss of primary carer, difficulties in swallowing tablets and adverse effects, all of which eventually affects adherence.
- Commitment and involvement of a responsible carer will help the treatment of a child successfully.
- Parents and other family members of children living with HIV may themselves be living with HIV; suboptimal HIV care and treatment for family members could result in suboptimal care for the child.
Reference
1. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach WHO 2016, 2nd Edition.
Nutrition in HIV-infected Infants and Children1,2
- It is important to identify malnutrition and growth faltering early. These are examined by nutritional assessment.
- Infants and children should intially undergo complete nutritional assessment, which includes evaluation of nutritional status, diet and symptoms and thereafter be weighed and have height measured at each visit and monitored with reference to WHO or national growth curves.
- Growth monitoring should also be integrated into the assessment of ART response.
- Further assessment should be performed to determine the cause, and plan appropriate response if poor growth is identified.
- HIV-infected children on or off ART who are symptomatic, have conditions requiring increased energy (e.g. TB, chronic lung disease, chronic opportunistic infections or malignancies), or have weight loss, or have evidence of poor growth, should be provided with 25–30% additional energy.
- HIV-infected children who are severely malnourished should be managed as per the guidelines for uninfected children and provided with 50–100% additional energy.
- HIV-infected children should receive one recommended daily allowance (RDA) of micronutrients daily. If this cannot be assured through the diet, or there is evidence of deficiency, then supplementation should be given.
- HIV-infected infants and children between 6 and 59 months of age should receive high-dose vitamin A supplementation every 6 months, as per the guidelines for uninfected children.
- HIV-infected children who have diarrhoea should receive zinc supplementation as a part of management, as per the guidelines for uninfected children.
References
1. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach WHO 2016, 2nd Edition.
2. Antiretroviral therapy of HIV infection in infants and children: towards universal access: recommendations for a public health approach – WHO 2010 revision.
Appendix
Appendix–1: WHO Clinical Staging of HIV Disease in Children
|
Clinical stage 1 |
|
Asymptomatic Persistent generalized lymphadenopathy |
|
Clinical stage 2 |
|
Unexplained persistent hepatosplenomegaly Recurrent or chronic upper respiratory tract infections (otitis media, otorrhoea, sinusitis, tonsillitis) Herpes zoster Lineal gingival erythema Recurrent oral ulceration Papular pruritic eruption Fungal nail infections Extensive wart virus infection Extensive molluscum contagiosum Unexplained persistent parotid enlargement |
|
Clinical stage 3 |
|
Unexplained moderate malnutritiona not adequately responding to standard therapy Unexplained persistent diarrhoea (14 days or more) Unexplained persistent fever (above 37.5°C, intermittent or constant, for longer than one 1 month) Persistent oral candidiasis (after first 6 weeks of life) Oral hairy leukoplakia Lymph node tuberculosis Pulmonary tuberculosis Severe recurrent bacterial pneumonia Acute necrotizing ulcerative gingivitis or periodontitis Unexplained anaemia (<8 g/dl), neutropaenia (<0.5 x 109/l) or chronic
thrombocytopaenia Symptomatic lymphoid interstitial pneumonitis Chronic HIV-associated lung disease, including bronchiectasis |
|
Clinical Stage 4 |
|
Unexplained severe wasting, stunting or severe malnutritionb not responding to standard therapy Pneumocystis (jirovecii) pneumonia Recurrent severe bacterial infections (such as empyema, pyomyositis, bone or joint infection, meningitis, but excluding pneumonia) Chronic herpes simplex infection (orolabial or cutaneous of more than 1 month’s duration or visceral at any site) Oesophageal candidiasis (or candidiasis of trachea, bronchi or lungs) Extrapulmonary tuberculosis Kaposi sarcoma Cytomegalovirus infection (retinitis or infection of other organs with onset at age more than 1 month) Central nervous system toxoplasmosis (after the neonatal period) HIV encephalopathy Extrapulmonary cryptococcosis, including meningitis Disseminated nontuberculous mycobacterial infection Progressive multifocal leukoencephalopathy Chronic cryptosporidiosis (with diarrhoea) Chronic isosporiasis Disseminated endemic mycosis (extrapulmonary histoplasmosis, coccidioidomycosis, penicilliosis) Cerebral or B-cell non-Hodgkin lymphoma HIV-associated nephropathy or cardiomyopathy |
a For children younger than 5 years, moderate malnutrition is defined as weight-for-height <–2 z-score or mid-upper arm circumference ≥115 mm to <125 mm.
b For children younger than 5 years of age, severe wasting is defined as weight-for-height <–3 z-score; stunting is defined as length-for-age/height-for-age <–2 z-score; and severe acute malnutrition is either weight for height <–3 z-score or mid-upper arm circumference <115 mm or the presence of oedema.
Appendix–2: Cipla’s Range of WHO and USFDA Approved Paediatric Antiretrovirals
|
WHO Prequalified ARVs |
USFDA Approved ARVs |
|
Lamivudine 50 mg / 5 ml oral solution |
Zidovudine Oral Solution, 50 mg / 5 ml |
|
Zidovudine 50 mg / 5 ml oral solution |
Lamivudine 10 mg / ml Oral Solution |
|
Nevirapine 50 mg / 5 ml oral suspension |
Abacavir oral solution, 20 mg / ml |
|
Abacavir oral solution, 20 mg / ml |
Lopinavir / Ritonavir oral solution 80 mg / 20 mg / ml |
|
Abacavir 60 mg tablets for oral suspension |
Abacavir tablets 60 mg tablets for oral suspension |
|
Lamivudine 30 mg tablets |
Abacavir / Lamivudine tablets for oral suspension, 60 mg / 30 mg |
|
Abacavir / Lamivudine dispersible tablets, |
Lamivudine / Zidovudine tablets 30 / 60 mg |
|
Nevirapine 50 / 100 mg dispersible tablets |
Nevirapine 50 / 100 mg tablets for oral suspension |
|
|
Lamivudine / Zidovudine tablets for oral suspension, 30 / 60 mg |
|
|
Lamivudine / Nevirapine / Zidovudine tablets for oral suspension 30 / 50 / 60 mg |
|
|
Ritonavir Tablets 25 / 50 mg |
|
|
Lopinavir and Ritonavir 40 mg / 10 mg Oral Pellets |
Appendix–3: Dosage and Recommendations1
General Principles
- Using an age-appropriate fixed-dose combination is preferable for any regimen if such a formulation is available.
- Oral liquid or syrup formulations should be avoided where possible. Dispersible tablets (or tablets for oral solution) are the preferred solid oral dosage forms, since each dispersible tablet can be made into liquid at the point of use.
- If suitable dispersible fixed-dose combinations are not available and oral liquids must be used, it is recommended that children be switched to a solid oral dosage form as soon as possible.
- Where children have to use adult formulations, care must be taken to avoid under dosing and overdosing. Using functionally scored tablets is preferable to ensure accurate dosing, especially if adult dosage forms are used. Splitting unscored tablets should be avoided, since uniform distribution of the active drug product cannot be assured in tablet fragments.
- Some tablets such as LPV/r or ATV heat-stable tablets are made in a special embedded matrix formulation (a proprietary melt extrusion technology that stabilizes drug molecules that are normally heat labile) and should not be cut, split, dissolved, chewed or crushed, since these products have variable bioavailability when not swallowed whole.
- Different dosing between morning and evening doses should be avoided if possible.
- Children have to be weighed at each clinic visit, and dose changes are required as children grow and/or change weight.
- Country programmes should consider the national regulatory status and local availability status and availability of specific dosage forms when developing national treatment recommendations for children.
- Research is ongoing for several ARV medications to establish dosing guidance for newborns, infants and young children. The age indications for each drug mentioned in the drug pages are based on current evidence and will be updated as new recommendations become available.
|
Drug |
Strength of tablets (mg) |
Number of tablets by weight band morning and evening |
Strength of adult tablet (mg) |
Number of tablets by weight band |
||||||||||
|
3.0–5.9 kg |
6.0–9.9 kg |
10.0–13.9 kg |
14.0–19.9 kg |
20.0–24.9 kg |
25.0–34.9 |
|||||||||
|
|
|
AM |
PM |
AM |
PM |
AM |
PM |
AM |
PM |
AM |
PM |
|
AM |
PM |
|
AZT/3TCa |
Tablet (dispersible) 60 mg/30 mg |
1 |
1 |
1.5 |
1.5 |
2 |
2 |
2.5 |
2.5 |
3 |
3 |
300 mg/ 150 mg |
1 |
1 |
|
AZT/3TC/ NVPa |
Tablet (dispersible) 60 mg/30 mg/50 mg |
1 |
1 |
1.5 |
1.5 |
2 |
2 |
2.5 |
2.5 |
3 |
3 |
300 mg/ 150 mg/200 mg |
1 |
1 |
|
ABC/3TC |
Tablet (dispersible) 60 mg/30 mg |
1 |
1 |
1.5 |
1.5 |
2 |
2 |
2.5 |
2.5 |
3 |
3 |
600 mg/ 300 mg |
0.5 |
0.5 |
|
ABC/3TC |
Tablet (dispersible) 120/60 mg |
0.5 |
0.5 |
0.5 |
1 |
1 |
1 |
1 |
1.5 |
1.5 |
1.5 |
600 mg/ 300 mg |
0.5 |
0.5 |
a For infants younger than 4 weeks of age, see Table 4 for more accurate dosing, which is reduced because of the decreased ability to excrete and metabolize medication. For infants who are at least 4 weeks of age but less than 3 kg, the immaturity of renal and hepatic pathways of elimination is less of a concern, but uncertainty still exists on the appropriate dosing of ARV drugs for preterm and low-birth weight infants.
|
Drug |
Strength of tablets (mg) |
Number of tablets or capsules by weight band once daily |
Strength of adult tablet (mg) |
Number of tablets by weight band |
||||
|
3.0–5.9 kg |
6.0–9.9 kg |
10.0–13.9 kg |
14.0–19.9 kg |
20.0–24.9 kg |
25.0–34.9 kg |
|||
|
EFVb |
Tablet (scored) 200 mg |
– |
– |
1 |
1.5 |
1.5 |
200 mg |
2 |
|
ABC/3TC |
Tablet (dispersible) 60/30 mg |
2 |
3 |
4 |
5 |
6 |
600 mg/300 mg |
1 |
|
ABC/3TC |
Tablet (dispersible) 120/60 mg |
1 |
1.5 |
2 |
2.5 |
3 |
600 mg/300 mg |
1 |
|
ATVc |
Capsules 100 mg |
– |
– |
1 |
2 |
2 |
300 mg |
2 (100 mg)dor 1 (300 mg) |
|
TDFe |
Oral powder scoops 40 mg/scoop |
– |
– |
3 |
– |
– |
300 mg |
1 (200 mg)d or 1 (300 mg) |
|
Tablets 150 mg or 200 mg |
– |
– |
– |
1 (150 mg) |
1 (200 mg) |
|||
a For infants younger than 4 weeks of age, see Table 4 for more accurate dosing, which is reduced because of the decreased ability to excrete and metabolize medication. For infants who are at least 4 weeks of age but less than 3 kg, the immaturity of renal and hepatic pathways of elimination is less of a concern, but uncertainty still exists on the appropriate dosing of ARV drugs for preterm and low-birth weight infants.
b EFV is not recommended for children younger than 3 years and weighing less than 10 kg. The United States Food and Drug Administration approved EFV for use for children younger than 3 years weighing more than 3.5 kg during the finalization of these guidelines (3.5–5.0 kg: two 50-mg capsules; 5.0–7.5 kg: three 50-mg capsules; 7.5–15.0 kg: one 200-mg capsule), but more data are urgently needed to inform recommendations for using EFV in this age group.
c ATV is only approved for use for children 3 months and older. ATV single-strength capsules should be administered with RTV 100 mg for all weight bands. The ATV powder formulation enables administration of ATV to infants and children as young as 3 months. Infants and children weighing 5–10 kg should be administered 200 mg of ATV powder (4 packets, 50 mg per packet) with 80 mg of RTV oral solution (5 ml).
d 200 mg should be used for weight 25.0–29.9 kg and 300-mg tablets for 30.0–34.9 kg.
e TDF is only approved for use for children 2 years and older. Target dose: 8 mg/kg or 200 mg/m2 (maximum 300 mg). The Paediatric Antiretroviral Working Group developed this guidance to harmonize TDF dosing with WHO weight bands and to reduce the numbers of strengths to be made available. The WHO generic tool was used based on the target dose provided by the manufacturer’s package insert. In accordance with the standard Paediatric Antiretroviral Working Group approach, dosing was developed ensuring that a child would not receive more than 25% above the maximum target dose or more than 5% below the minimum target dose.
|
Drug |
Strength of tablets (mg) or oral liquid (mg/ml) |
Number of tablets or ml by weight-band morning (AM) and evening (PM) |
Strength of adult tablet (mg) |
Number of tablets by weight band |
||||||||||
|
|
|
3.0–5.9 kg |
6.0–9.9 kg |
10.0-13.9 kg |
14.0-19.9 kg |
20.0-24.9 kg |
|
25.0–34.9 kg |
||||||
|
|
|
AM |
PM |
AM |
PM |
AM |
PM |
AM |
PM |
AM |
PM |
|
AM |
PM |
|
Solid formulations |
||||||||||||||
|
AZT |
Tablet (dispersible) 60 mg |
1 |
1 |
1.5 |
1.5 |
2 |
2 |
2.5 |
2.5 |
3 |
3 |
300 mg |
1 |
1 |
|
ABC |
Tablet (dispersible) 60 mg |
1 |
1 |
1.5 |
1.5 |
2 |
2 |
2.5 |
2.5 |
3 |
3 |
300 mg |
1 |
1 |
|
NVPb |
Tablet (dispersible) 50 mg |
1 |
1 |
1.5 |
1.5 |
2 |
2 |
2.5 |
2.5 |
3 |
3 |
200 mg |
1 |
1 |
|
LPV/rc |
Tabletd 100 mg/ 25 mg |
– |
– |
– |
– |
2 |
1 |
2 |
2 |
2 |
2 |
100 mg/ 25 mg |
3 |
3 |
|
Pelletse 40 mg/ 10 mg |
2 |
2 |
3 |
3 |
4 |
4 |
5 |
5 |
6 |
6 |
100 mg/ 25 mg |
3 |
3 |
|
|
DRVf |
Tablet 75 mg |
– |
– |
– |
– |
3 |
3 |
5 |
5 |
5 |
5 |
|
|
|
|
RAL |
Chewable tablets 25 mg |
– |
– |
– |
– |
3 |
3 |
4 |
4 |
6 |
6 |
400 mg |
1 |
1 |
|
Chewable tablets 100 mg |
– |
– |
– |
– |
– |
– |
1 |
1 |
1.5 |
1.5 |
400 mg |
1 |
1 |
|
|
Granulesg (100 mg/sache) |
0.25 |
0.25 |
0.5 |
0.5 |
– |
– |
– |
– |
– |
– |
|
– |
– |
|
|
Liquid formulations |
||||||||||||||
|
AZT |
10 mg/ml |
6 ml |
6 ml |
9 ml |
9 ml |
12 ml |
12 ml |
– |
– |
– |
– |
– |
– |
– |
|
ABC |
20 mg/ml |
3 ml |
3 ml |
4 ml |
4 ml |
6 ml |
6 ml |
– |
– |
– |
– |
– |
– |
– |
|
3TC |
10 mg/ml |
3 ml |
3 ml |
4 ml |
4 ml |
6 ml |
6 ml |
– |
– |
– |
– |
– |
– |
– |
|
NVPb |
10 mg/ml |
5 ml |
5 ml |
8 ml |
8 ml |
10 ml |
10 ml |
– |
– |
– |
– |
– |
– |
– |
|
LPV/rc |
80/20 mg/ml |
1 ml |
1 ml |
1.5 ml |
1.5 ml |
2 ml |
2 ml |
2.5 ml |
2.5 ml |
3 ml |
3 ml |
– |
– |
|
|
DRVf |
100 mg/ml |
– |
– |
– |
– |
2.5 ml |
2.5 ml |
3.5 ml |
3.5 ml |
– |
– |
|
|
|
a For infants younger than 4 weeks of age, see Table 4 for more accurate dosing, which is reduced because of the decreased ability to excrete and metabolize medication. For infants who are at least 4 weeks of age but less than 3 kg, the immaturity of renal and hepatic pathways of elimination is less of a concern, but uncertainty still exists on the dosing of ARV drugs for preterm and low-birth-weight infants.
b NVP dose escalation with half dose for 2 weeks when initiating ART is still recommended to avoid toxicity from high initial NVP levels. However, secondary analysis from the (CHAPAS)-1 trial recently suggested that younger children have a lower risk of toxicity, and consideration can be given to starting with a full dose.
c LPV/r liquid requires a cold chain during transport and storage. The LPV/r heat-stable tablet formulation must be swallowed whole and should not be split, chewed, dissolved or crushed.
d The adult 200/50 ml tablet could be used for children 14.0–24.9 kg (1 tablet in the morning and 1 tablet in the evening) and for children 25.0–34.9 kg (2 tablets in the morning and 1 tablet in the evening).
e The LPV/r pellets formulation should not be used for infants younger than 3 months.
f DRV must be administered with 0.5 ml of RTV 80 mg/mL oral suspension if the child weighs less than 15 kg and with RTV 50 mg solid formulation for children weighing 15–30 kg.
g RAL granules are approved for use for children as young as 4 weeks, but the feasibility and acceptability of such formulations has not been widely investigated, and concerns have been raised regarding administration in resource-limited settings. The bioequivalence of RAL chewable tablets dispersed in liquid is currently being explored, and more guidance will be provided as soon as additional evidence becomes available.
|
Drug |
Strength of oral liquid (mg/ml) |
2–3 kg |
3–4 kg |
4–5 kg |
|
AZT |
10 mg/mL |
1 mL |
1.5 mL |
2 mL |
|
NVP |
10 mg/mL |
1.5 mL |
2 mL |
3 mL |
|
3TC |
10 mg/mL |
0.5 mL |
0.8 mL |
1 mL |
|
LPV/rb |
80/20 mg/mL |
0.6 mL |
0.8 mL |
1 mL |
a There is limited experience with initiating treatment among newborns living with HIV <2 weeks of age, with few pharmacokinetic data to fully inform accurate dosing for drugs other than AZT during a time that renal and liver functioning is rapidly maturing, and LPV/r solution should not be given to infants aged <2 weeks, making management of HIV treatment in newborns challenging. In addition, reliable pharmacokinetic data for preterm infants are available only for AZT, with uncertainty of dosing for NVP and 3TC; LPV/r solution should not be given in preterm infants until they have reached 42 weeks of gestational age. This guidance will be updated when more evidence is available from ongoing trials.
b Do not use LPV/r solution for infants <2 weeks of age. LPV/r pellets should not be used for infants younger than 3 months.
|
Drug |
Strength of tablet or oral liquid (mg or mg/5ml) |
Number of tablets or millilitres by weight band once daily
|
Strength of adult tablet (mg) |
Number of tablets by weight band |
||||
|
|
|
3.0–5.9 kg |
6.0–9.9 kg |
10.0– 13.9 kg |
14.0– 19.9 kg |
20.0– 24.9 kg |
|
25.0–34.9 kg |
|
Isoniazid |
100 mg |
0.5 |
1 |
1.5 |
2 |
2.5 |
300 mg |
1 |
|
Co-trimoxazole |
Suspension 200/40 per 5 ml |
2.5 ml |
5 ml |
5 ml |
10 ml |
10 ml |
– |
– |
|
Tablets (dispersible) 100/20 mg |
1 |
2 |
2 |
4 |
4 |
– |
– |
|
|
Tablets (scored) 400/80 mg |
– |
0.5 |
0.5 |
1 |
1 |
400 mg/ 80 mg |
2 |
|
|
Tablets (scored) 800/160 mg |
– |
– |
– |
0.5 |
0.5 |
800 mg/ 160 mg |
1 |
|
|
Isoniazid + co-trimoxazole + B6a |
Tablets (scored) 300 mg/960 mg/25 mg |
– |
– |
– |
0.5 |
0.5 |
960 mg/ 300 mg/ 25 mg |
1 |
|
a This formulation is currently awaiting regulatory approval, and a scored tablet (480 mg/150 mg/12.5 mg) is also being developed. |
||||||||
|
Drug |
Strength of dosage form (mg) |
Number of tablets or sprinkle capsules or sachets by weight band |
|||||||||||
|
3.0–5.9 kg |
6.0–9.9 kg |
10.0–13.9 kg |
14.0–19.9 kg |
20.0–24.9 kg |
25.0–34.9 kg |
||||||||
|
AM |
PM |
AM |
PM |
AM |
PM |
AM |
PM |
AM |
PM |
AM |
PM |
||
|
ABC + 3TC + LPV/r |
30 mg/15 mg/40 mg/10 mg |
2 |
2 |
3 |
3 |
4 |
4 |
5 |
5 |
6 |
6 |
– |
|
|
AZT + 3TC + LPV/r |
30 mg/15 mg/40 mg/10 mg |
2 |
2 |
3 |
3 |
4 |
4 |
5 |
5 |
6 |
6 |
– |
|
|
DRV/r |
120 mg/20 mg |
– |
– |
– |
– |
2 |
2 |
3 |
3 |
3 |
3 |
4 |
4 |
|
ATV/ra |
100 mg/33 mg |
– |
– |
1 |
2 |
2 |
– |
||||||
|
ABC + 3TC + EFV |
150 mg/75 mg/150 mg |
|
|
1.5 |
2 |
2.5 |
3 |
||||||
|
a Dosing for ATV/r at 14.0–19.9 kg has been adjusted since previous versions of this annex to address concerns of potential under dosing of ATV. |
|||||||||||||
Reference
1. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach WHO 2016, 2nd Edition.