Section 1: Epidemiology of Osteoarthritis1-5
Osteoarthritis (OA) is the most common joint disease in the world with a higher prevalence in women than man. The prevalence increases dramatically with age, with up to 45 % of women over 65 years having symptoms and up to 70 % showing radiological evidence of OA, often impacting mobility.
According to the United Nations, by 2050 people aged over 60 will account for more than 20% of the world’s population. Of that 20%, a conservative estimate of 15% will have symptomatic OA, and one-third of these people will be severely disabled.
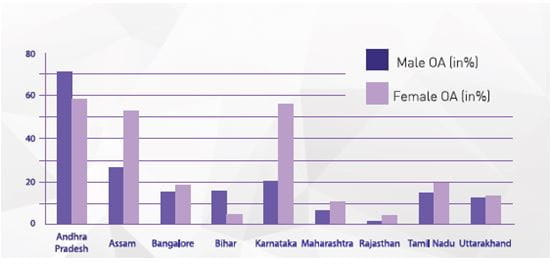
In India, OA is the most frequent joint disease with a prevalence of 22% to 39%. According to the results of COPCORD study, published by Haq et al, OA was a predominant musculoskeletal disorder and the knee was the commonest site of musculoskeletal pain, among Indians, more specifically in rural India. Prevalence in women was found to be twice that in men, in the same study. (See Fig 1 for gender-wise distribution of OA in different states in India.)
Adapted from Azad, CS, et al Osteoarthritis in India: An epidemiological aspect, Int J of Recent Sci Res, 2017; 8(10) : 20918-20922.
|
Section 2: Risk Factors for OA6,7
Adapted from Azad CS et al. Osteoarthritis in India. An epidemiological aspect, Int J of Recent Sci Res, 2017:8(10):20918-20922.
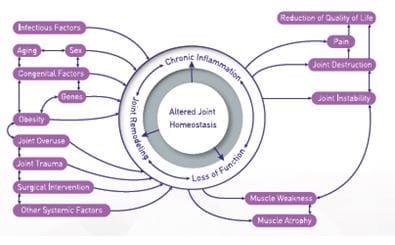
The risk factors can be either systemic such as genetics, dietary intake, estrogen use, and bone density or local such as muscle weakness, obesity, and joint laxity.
Age: Age plays a multifactorial role as it results in oxidative damage, thinning of cartilage, muscle weakening, and a reduction in proprioception
Gender: Females are more susceptible to hip, knee and hand OA than men, and the incidence increases around menopause
Obesity: For every 5-unit increase in BMI, the associated increased risk of knee OA was 35%, with the magnitude of the association significantly stronger for women than men.
Genetics: Genetic factors account for 60% of hand and hip OA and 40% of knee OA.
Diet: Low level of vitamins D, C and K may lead to increased risk of OA
Pathophysiology of OA -Current Concepts
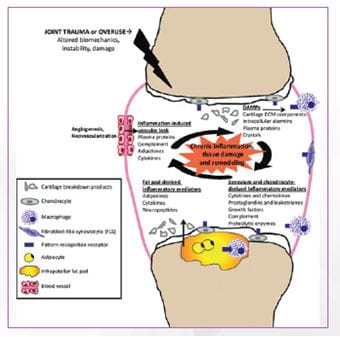
- Osteoarthritis (OA) is a whole joint disease, involving structural alterations in the hyaline articular cartilage, subchondral bone, ligaments, capsule, synovium, and periarticular muscles.
- The complex pathogenesis of OA involves mechanical, inflammatory, and metabolic factors, leading to structural destruction of the joint.
- Recent research indicates that the disease is, in fact, a dynamic alteration arising from an imbalance between repair and destruction of joint tissues.8
DAMPs, Damage-associated molecular patterns; ECM, extracellular matrix
Adapted from: Sokolove, J. and C. M. Lepus, Role of inflammation in the pathogenesis of osteoarthritis: Latest findigs and interpretations. Ther Adv Musculoskelet Dis, 2013,5(2):p.77-94.
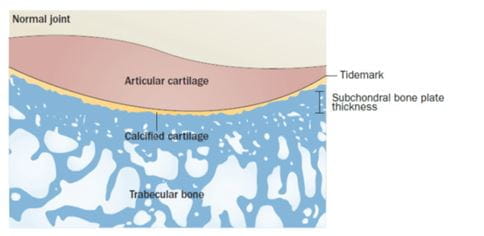
Changes in the cartilage10-12 (Figure 3 and 4)
Type II collagen, forms a meshwork providing the cartilage with tensile strength. Aggrecan and other proteoglycans are embedded within this mesh draw water into the cartilage, providing compressive resistance. Cartilage architecture and biochemical composition is regulated by chondrocytes in response to changes in their chemical and mechanical environment.
During Osteoarthritis:
- Cartilage composition changes increasing its susceptibility to disruption by physical forces and the cartilage loses its integrity.
- Initially, erosions are only at the surface; then later more deep cartilage fissures are followed by the expansion of the calcified cartilage zone.
- Hypertrophic chondrocytes inhibit the synthesis of the matrix constituents by generating matrix degradation products and proinflammatory mediators such as cytokines, including interleukin 1β, interleukin 6, and tumour necrosis factor (TNF) α, and matrix-degrading enzymes.
- The cytokines increase the MMPs synthesis, and inhibit major physiological inhibitors of these enzymes.
Changes in subchondral bone11,13
In the subchondral cortical bone:
- Bone turnover is increased at the interface between the calcified cartilage below the tidemark and the underlying trabecular bone
- Vascular invasion takes place, going from the subchondral bone, through the tidemark, and into the cartilage.
- The bone remodelling and repair is also associated with the development of subchondral bone marrow lesions.
- The osteophytes that develop at the joint margins through reactivation of endochondral ossification are strongly affected by inflammatory biological factors, but also by overload and abnormal joint kinematics.
Changes in synovium13
Synovitis is a common feature of osteoarthritis, even in early disease.
Following features are visible in established osteoarthritis:
- Proliferation of synoviocytes
- Tissue hypertrophy
- Reduced lubrication as Synoviocytes are responsible for endogenous lubricant synthesis such as hyaluronic acid and lubricin
- Synoviocytes, also release inflammatory mediators (IL-1beta and TNF-alpha) and degradative enzymes, probably secondary to inflammatory mediators and cartilage matrix molecules released during an initial insult to the joint. Subsequently, the synovial tissue drives progressive joint degeneration in a positive feedback cycle.
Changes in endogenous Hyaluronic acid (HA) in patients with OA14-16
HA is a glycosaminoglycan molecule and is one of the main components of normal synovial fluid and cartilage extracellular matrix, providing lubrication, shock absorption, and viscoelastic properties.
· Studies suggest that with aging there is a 33% to 50% reduction in HA concentration in synovial fluid.
Synovial fluid isolated from joints affected by OA is lower in elasticity and viscosity than that obtained from normal, healthy joints as a consequence of reductions in the concentration and molecular weight of HA by free radicals, inflammatory cytokines, and cleavage enzymes.
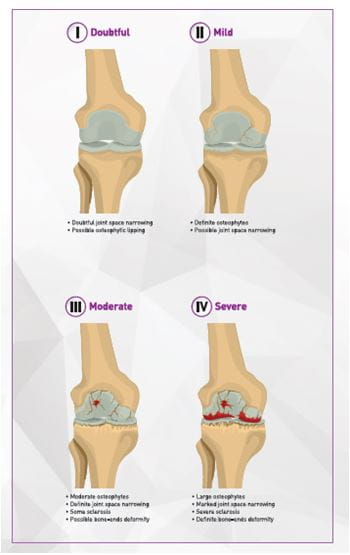
Stages of OA
The progression of osteoarthritis is divided into four stages. (Figure 4)
Image adapted from AADS
Mark D. Kohn, Adam A. Sassoon, and Navin D. Fernando. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin Orthop Relat Res. 2016 Aug;474(8):1886-1893.
Adapted from Burr DB, Gallant MA. Bone remodelling in osteoarthritis.
Nat Rev Rheumatol. 2012 Nov;8(11):665-73.
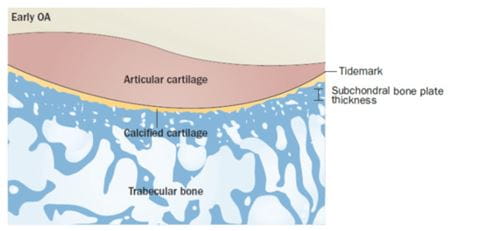
In early-stage OA
- The subchondral plate becomes thinner as a consequence of an increased remodelling rate
- Cancellous bone is lost as the trabecular plates become thinner and more rod-like.
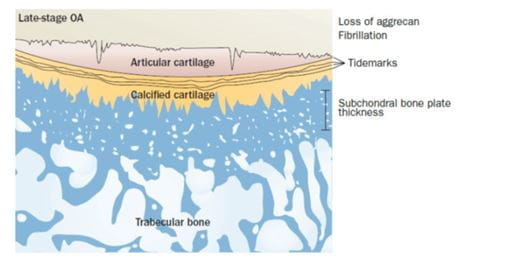
In late-stage disease
- The subchondral plate thickens
- The subchondral cancellous bone remains osteopenic.
- The calcified cartilage begins to advance into the articular cartilage in late-stage disease creating an even thicker mineralized plate and reducing the thickness of the non-mineralized articular cartilage which cannot replace itself.
- This is accompanied by a loss of aggrecan beginning superficially in the articular cartilage (shown by a change in stain color), and surface fibrillation.
- The sum result of these changes is subchondral sclerosis (that includes both the subchondral plate and calcified cartilage) and thinner, more fibrillated articular cartilage.
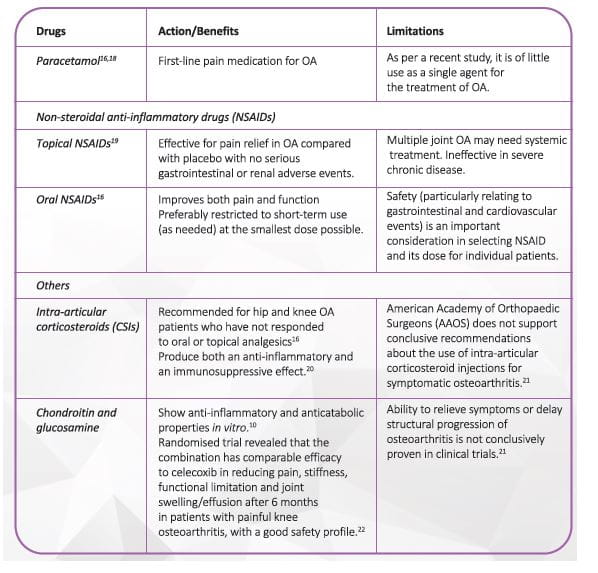
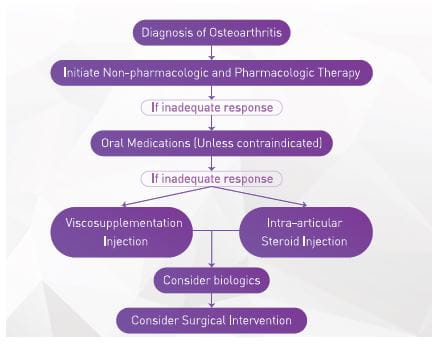
Section 3: OA Management16-17
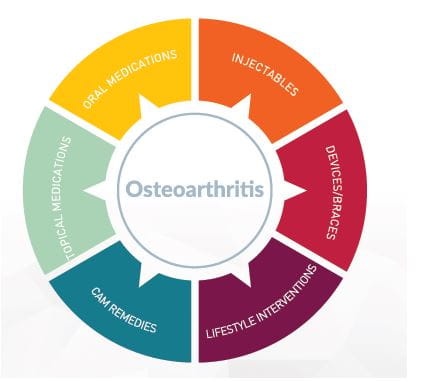
The goals of OA management are to alleviate pain, minimize loss of physical function and delay surgery. Several pharmacological and non-pharmacological interventions, in combination, help achieve these goals (Figure 6).
CAM, complementary and alternative medicine
There are six types of interventions that should be considered for the treatment of OA:
- Lifestyle interventions
- Oral medications
- Injectable medications
- Complementary and alternative remedies
- Braces/devices
- Surgical therapy
Patient education and self-management, exercise including physiotherapy or swimming, weight loss if overweight or obese, and walking aids are widely recommended and seen as first-line treatment. 17
The Table below enlists the commonly used pharmacological agents in OA.
Several guidelines and recommendations are available for treatment of OA (Figure 7)
Total joint replacement surgery16,25-29
Total joint replacement has changed the scenario of OA management. Total hip and knee replacements are most frequently performed definitive treatment options in end-stage OA.
The characteristics of end-stage osteoarthritis when surgery is recommended include joint pain, which disrupts normal sleep patterns and causes a severe reduction in capable walking distance and marked restriction of daily activities. Though total joint replacement is a successful procedure for pain relief and functional restoration, it may not be feasible in all patients.
Significant medical disease where the risk of surgery outweighs the expected benefit, may have poor prognosis after surgery.
Some such conditions include:
- Systemic infections
- Obesity may pose significant risk in performing surgery
- Psychiatric disease
- Dementia
- Smoking
- High risk patients e.g. very elderly individuals with multiple comorbidities
Alternative lower risk therapies that can delay or obviate TKR are valuable to those who are poor candidates for surgery or wish to avoid TKR as long as possible. As per AAOS estimation, 10 percent of patients undergoing knee replacements will need a revision at some point. The younger patients are when they have the first surgery and the longer they live afterward, the more likely they will be to need revision surgery. Hence joint replacement is not advocated in too young patients and should be delayed as long as possible. In a developing country like India this imposes a lot of economic burden as OA expenditure is maximum in patients undergoing knee and hip surgery; almost half of the total cost burden of the disease.
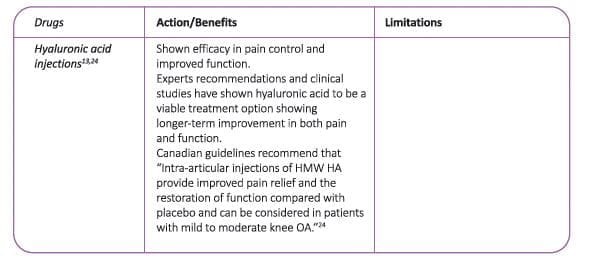
Viscosupplementation with hyaluronic acid (HA) injections, have been found to be safe and effective treatment to improve pain, function, and longevity of the knee with a potential to delay or obviate TKR for up to 3 years. It is also viable option for patients who cannot afford the total joint replacement surgery.
The concept of viscosupplementation has evolved over the years, with innovative formulations which enhance the safety and efficacy of HA in OA patients.
Section 4: Viscosupplementation
Use of intra-articular hyaluronic acid (IA-HA) to slow progression30
In the early 90s EA Balazs introduced the concept of viscosupplementation with the hypothesis that intra-articular (IA) injections of exogenous hyaluronic acid (HA) could restore visco-elasticity of the osteoarthritic synovial fluid (SF).
After more than 20 years of use, VS has been recognized as a safe and effective treatment of knee OA.
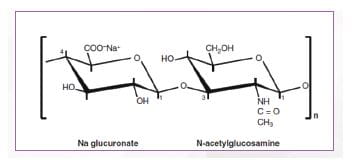
HA -Structure31
HA is a naturally occurring linear polysaccharide consisting of long, unbranched chain of two repeating sugar units. Each sugar unit is identical containing two linked monosaccharides (D-glucuronic acid and N-acetyl-D-glucosamine). The molecular weight of the molecule is determined by the number of disaccharide units linked together. The linear HA molecules form a flexible molecular network that binds water, providing organs structure, firming tissues, and acting as the body’s lubricant. (Figure 8)
n = degree of polymerization (Adapted from Agerup B, et al. BioDrugs. 2005;19(1):23-30.
|
HA is an unusual biomolecule HA is an unusual biomolecule, in that its chemical structure is identical regardless of the species in which it is found. The lack of structural differences between species ensures that inter-species biocompatibility is maintained.
|
Endogenous HA in the joints31,32
- HA is produced by chondrocytes and synoviocyte cells, and is a major component of human synovial fluid.
- Its viscoelastic properties allow it to serve as both a joint lubricant and a shock absorber, protecting cartilage from damage caused by heavy loads placed on weight-bearing joints.
- HA can form a pericellular coat around cells, interact with proinflammatory mediators, and bind to cell receptors, such as cluster determinant (CD)44 and receptor for hyaluronate-mediated motility (RHAMM), where it modulates cell proliferation, migration, and gene expression
- All these physicochemical and biologic properties of HA have been shown to be molecular weight (MW) dependent.
- The molecular weight of HA in healthy synovial fluid is reported as being approximately 7 million Daltons (MDa).
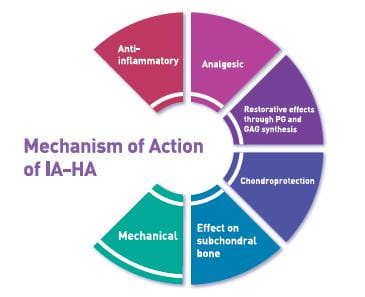
Mechanism of action of IAHA15,34,35
Viscosupplementation consists of injection of exogenous HA into diarthrodial joints, with the aim of restoring the rheological properties of the synovial fluid, thereby producing mechanical, analgesic, anti-inflammatory and chondroprotective effects.
IAHA provides the following benefits:
Mechanical effects
Osteoarthritic knees are reported to have higher friction within the joint space than healthy knees, which is counteracted by the joint lubrication capabilities that HA possesses
- The viscous nature of HA treatment is shown to lubricate the joint capsule, preventing degeneration through decreased friction.
- HA further protects the joint capsule through beneficial shock absorption effects by absorbing pressure and vibration within the joint that otherwise may lead to chondrocyte degradation
Chondroprotective effects
The chondroprotective effects of IA-HA is due to reduction in chondrocyte apoptosis while increasing chondrocyte proliferation by restricting cytokines.
Activated synoviocytes in OA joints, produce a variety of cytokines and enzymes, such as interleukin (IL)-β1, IL-6, IL-8, TNF-alpha, metalloproteinases, aggrecanases and nitric oxide (NO). HA acts as an important modulator of these cytokines and enzymes, through interaction with CD44 receptors that are present in synoviocytes. 35 It results in reduction in IL-1β-induced oxidative stress, through inhibition of NO production within the synovium, and increased heat shock protein 70 (Hsp70) overexpression.
IA-HA protects the cartilage by:
- Reducing disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) expression, which are involved in the cleavage of important synovial components, including aggrecan, versican, and brevican
- Reducing prostaglandin E2 (PGE2) synthesis
- Increasing cartilage volume as was observed by means of imaging examinations
Biopsies performed before and after viscosupplementation showed that, six months later, the surface layer had been reconstituted, with better matrix quality, higher chondrocyte density and greater numbers of organelles inside. Diminished loss of joint space was observed one year after the procedure.
Proteoglycan and glycosaminoglycan (GAG) synthesis
As OA progresses, intrinsic proteoglycan and GAG concentrations decline within the cartilage.
- IA-HA treatment stimulates proteoglycan synthesis, delaying the progression of OA
- IA-HA suppresses degradation of aggrecan and promotes intrinsic aggrecan development through CD44 and intercellular adhesion molecule (ICAM)-1 binding effects
- It mobilizes newly synthesized proteoglycan to the outer chondrocyte matrix, potentially providing protection from degradation.
- IA-HA treatment is also shown to increase endogenous GAG production.
Anti-inflammatory effects
- IA-HA suppresses IL-1β expression by binding CD44 receptor, thereby down-regulating MMPs
- Further suppression of pro-inflammatory mediators IL-8, IL-6, PGE2, and tumor necrosis factor (TNFα) provides anti-inflammatory effects
Structural benefit on the subchondral bone
- IA-HA affects the subchondral bone through suppression of MMP-13 and IL-6 via CD44 binding, which potentially prevents abnormal osseous tissue metabolism.
- The structural benefit of viscosupplementation has been seen through second-look arthroscopy, performed one year after treatment started, in which the joint surface was seen to have a better visual appearance, compared with a placebo group.
Analgesic effects
IAHA has an analgesic effect, thereby diminishing nerve impulses and the sensitivity of nociceptive nerve ends.
Fig 9 summarises the mechanisms of action of IA-HA on the various parts of the joint.
GAG, Glycosaminoglycans; PG, prostaglandin
Viscosupplementation delays surgery –bridge therapy26,29,36
Trials with IA HA have shown multiple benefits including reduction in pain for several months in contrast to the short-term benefits of intra-articular corticosteroids (IA-CS) on pain.
These pharmacological properties of HA help in delaying surgery or can help patients in whom surgery is thought to be of high risk e.g. very elderly individuals with multiple comorbidities.
A recent retrospective and observational study on real-world knee OA patients in France demonstrated time-to-TKR of 217 (±10) days after 7.5 years of diagnosis compared to patients who did not receive HA (p-value<0.0001).
In another retrospective analyses by Dasa V et al showed a good safety profile and led to high proportions of patients without TKR 3 years after treatment initiation.
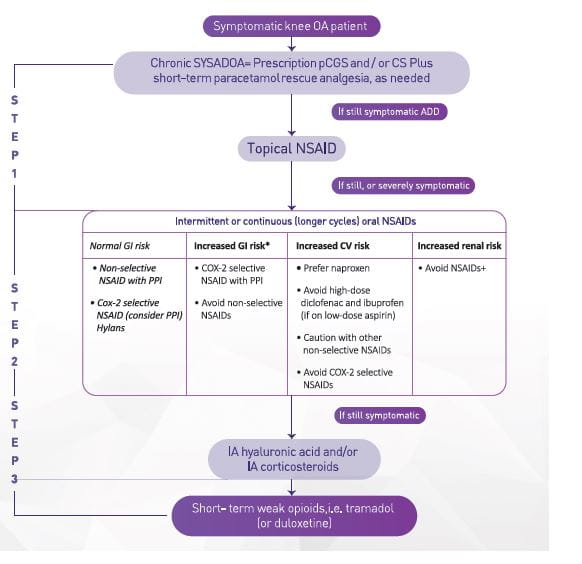
Place in therapy - What guidelines recommend24,36,37
The use of IA HA in knee OA patients with mild-moderate disease, and for more severe patients wishing to delay TKR surgery, is recommended by the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) task force.
- Nonetheless, IA HA is an effective and safe treatment for long-term management of knee OA and may be a cost-effective treatment. (Figure 10)
- IA HA should only be administered in knee OA once the acute inflammatory flare has settled.
- Although the treatment effect of IA HA is comparable with NSAIDs, IA HA is positioned later in the treatment algorithm, unless NSAIDs are contraindicated, due to the requirement for repeat injections usually performed by a trained, specialized practitioner (either orthopedic surgeon or rheumatologist).
⁎Including use of low-dose aspirin; †With glomerular filtration rate <30 cc/min; caution in other cases, COX-2, cyclooxygenase-2; CS, chondroitin sulfate; CV, cardiovascular; GI, gastrointestinal; IA, intra-articular; NSAID, non-steroidal anti-inflammatory drug; pCGS, patented crystalline glucosamine sulfate; PPI, proton pump inhibitor; SYSADOA, symptomatic slow-acting drugs in OA.
The National Authority for Health in France, the Haute Autorite de Sante (HAS), currently defines HA as a treatment option, more specifically for patients who are intolerant to and/or have an inadequate response to NSAIDs.
Also the 2019 Canadian guidelines recommend that intra-articular injections of HMW HA can be considered in patients with mild to moderate knee OA as they provide improved pain relief and the restoration of function.
Section 5: Enhancing Properties of IA-HA for Maximising Efficacy
There are numerous types of viscosupplementation approved for use in OA. Each formula differs by its molecular weight, molecular structure, half-life, concentration, dosing frequency, and injection volume. 13
When attempting to optimize the clinical profile of an HA preparation, four key attributes must be considered: 33
- Residence time (half-life) within the joint,
- Biocompatibility (safety),
- Rheologic properties, and
- The density of HA (this affects the administered dose).
This is represented in Figure 11
These four characteristics are clearly determined by the physical and chemical properties of the HA formulation. 33
Types of HA preparations15,31,38,39
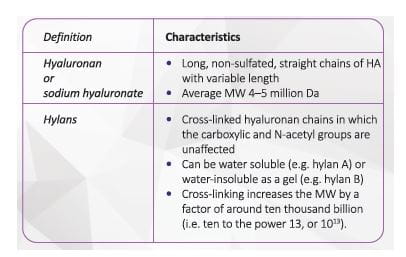
There are two broad types of HA preparations which differ in the above-mentioned characteristic: hyaluronans (unmodified hyaluronic acid) and hylans. 33
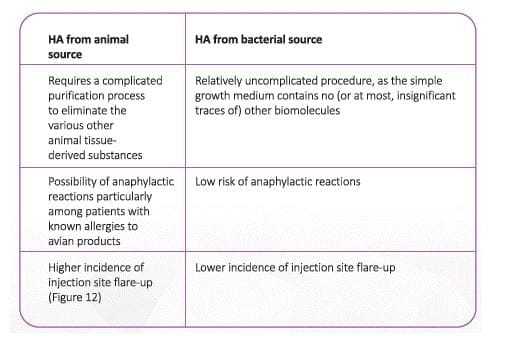
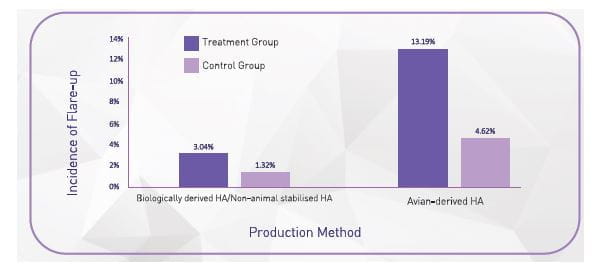
Hyaluronan is extracted from two types of sources: animal and non-animal. A non-animal source of HA, with cross
linking, has superior tolerability to animal /avian source HA
(Table 1) 41
Adapted from Altman, RD et al Product Differences in Intra-articular Hyaluronic Acids for Osteoarthritis of the Knee, Am J Sports Med. 2016 Aug;44(8):2158-65
Need for improvement in Hyaluronans for OA31
Hyaluronans are low/medium molecular weight (0.6–1.55 MDa) fractions of HA obtained from animal-derived raw materials (often rooster combs or bovine vitreous).
The limitations of these preparations include:
- Short residence time within the knee joint
Hyaluronans are readily degraded by free radicals, and because of their unbranched nature, even a small number of breaks can result in a profound decrease in their molecular size reducing residence time up to <24 hours within the knee joint.
- Concerns over
- A decrease in molecular size reduces the viscoelastic properties of lubrication and shock absorption. Lower molecular size also means, multiple injections.
The shift from Hyaluronans to Hylans31
There are several disadvantages of multiple injection preparations.
- A dosage of three to five injections per course of treatment for adequate pain relief severely limits patient convenience.
- The risk of adverse events, including injection-related infection, is also increased when more injections are administered.
Therefore, a decrease in the number of injections should improve the safety and tolerability of viscosupplementation, while also increasing convenience and, potentially, cost-effectiveness.
Section 6: Hylans
Hylans are high molecular weight, chemically cross-linked derivatives of hyaluronans aimed at increasing the intra-articular residence of hyaluronans. 31
Cross-linking provides stability and improves functionality of the gels. 40
Crosslinking is done by several methods such as water-soluble carbodiimide crosslinking, polyvalent hydrazide crosslinking, divinyl sulfone crosslinking, disulfide crosslinking, BDDE and formaldehyde crosslinking, and photo-crosslinking through glycidyl methacrylate-HA conjugation.41
Clinical and biocompatibility data from longer than 15 years support the favourable clinical safety profile of BDDE-crosslinked HA and its degradation products.42
Studies have consistently shown that high molecular weight preparations (approximately 6 MDa) had longer half-life compared with preparations of low molecular weight (approximately 100 000Da). 31
High molecular weight preparations have shown to have a superior binding to CD44 receptor, thereby having a superior mechanism of action compared to lower molecular weight HA- providing a better inhibition of inflammatory cytokines, a greater effect on proteoglycan synthesis, and better friction reduction due to its viscous properties.39
Non-animal stabilised HA31,43,44
Non-animal stabilised HA is HA produced by bacterial synthesis, which is biocompatible, as well as cross linked, to increase the molecular weight and therefore the residence time in the joints.
Non-animal stabilised HA is produced by a three-step process:
Step 1: Production
- In the production of non-animal stabilised HA, non-pathogenic bacteria (Streptococcus zooepidemicus ) are cultured in a simple nutrient medium.
- HA is produced within the cellular membrane of the bacteria and exported directly into the surrounding medium.
- HA (MW ~1 MDa) is extracted from the growth medium and purified such that is indistinguishable from endogenous HA.
- Isolation of pure HA following bacterial synthesis is relatively uncomplicated, as the simple growth medium contains no (or at most, insignificant traces of) other biomolecules.
Step 2: Stabilization
- Purified HA is dispersed into a solution containing 1, 4-butanediol diglycidyl ether.
- This cross-linking agent reacts with hydroxyl groups of HA, in carefully controlled and monitored conditions to ensure an optimum crosslinking of approximately 0.5–1.0% (approximately 1 in every 100 disaccharide units is joined to another unit and no additional modifications occur).
- The process joins the HA molecules to one another, so that it forms a three- dimensional gel.
- Each gel bead is effectively one enormous molecule of HA, which increases the molecular weight by a factor of around ten thousand billion (i.e. ten to the power 13, or 1013).
- A heavier crosslinking (20%) affects the biocompatibility and inhibits the recognition by endogenous HA receptors.
- The crosslinking due to increased molecular weight also improves the residential time of HA.
- Crosslinking preserves the hydration effect and slightly changes the rheological parameters.
Step 3: Sterilization
- Stabilized non-animal stabilised HA is manufactured under aseptic environment and steam-sterilized before packaging in order to achieve maximum safety.
Non-animal stabilised HA --Advantage in OA15,31,45
Non-animal stabilised HA offers advantages, with respect to all the four key attributes of IA-HA:
Intra-Articular Residence Time
- Non-animal stabilised HA possesses greater resistance to degradation within the body, and consequently has an increased intra-articular residence time.
- As part of an entangled network, HA molecules are prevented from coming into free contact with the cells that naturally degrade HA, and hence resists degradation.
- The true half-life of non-animal stabilised HA is believed to be 4 weeks in contrast to HMW preparations (13.2 hours for 6 MDa and 10.2 hours for 10 MDa)
Biocompatibility
- The non-animal source of HA lowers the risk of contamination by any molecules of animal origin.
Rheologic Properties
- Studies have shown that the rheologic properties of non-animal stabilised HA are superior to those of existing HA preparations.
Density of HA
- The physical structure of the non-animal stabilised HA gel also produces an increased density of HA compared with other HA preparations. The dose of HA delivered by each injection of non-animal stabilised HA is 60mg (3mL; 20 mg/mL HA), compared with typically 10–30mg per injection (3–10 mg/mL HA) for the other HA preparations.
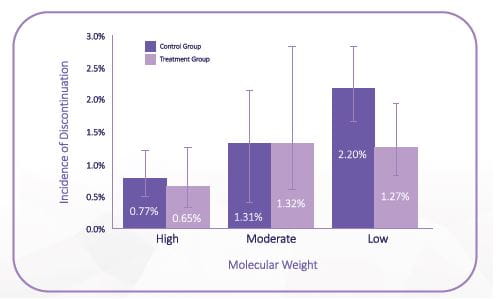
Overall enhanced tolerability
High molecular weight (HMW) HA, have been shown to have better tolerability as compared to low molecular weight HA, as fewer patients discontinued due to treatment related adverse events in the HMW-HA. (Figure 13)
High molecular weight, >3000 kDa; low molecular weight, ≤1500 kDa; moderate molecular weight, >1500 but <3000 kDa (Adapted from Altman, RD et al Product Differences in Intra-articular Hyaluronic Acids for Osteoarthritis of the Knee, Am J Sports Med. 2016 Aug;44(8):2158-65
Clinical benefits of non-animal stabilised HA in OA
The major therapeutic goals for the treatment of knee OA are to decrease joint pain and stiffness, improve function and QoL, and delay the need for TKA.
Based on the clinical outcomes in a recent review of clinical studies, once weekly administration of non-animal stabilised HA offered the following benefits in OA patients:
- Significant reductions from baseline pain, as well as improvements in physical function and joint stiffness from baseline levels, were observed 24- 26 weeks after a single injection of non-animal stabilised HA
- Self-reported enhancements in QoL following non-animal stabilised HA treatment were also found to be significant over an extended time frame.
- Importantly, non-animal stabilised HA was found to have a favourable safety and tolerability profile, with a low incidence of reported, transient treatment related adverse events (TRAEs).
Section 7: Hyaluronic Acid Clinical Studies
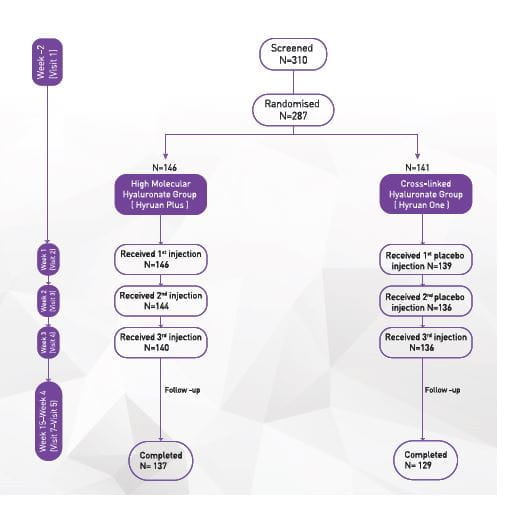
Study 146
Objective
To compare efficacy and safety of intra-articular BDDE-crosslinked hyaluronic acid injections (Hyruan One) once versus intra-articular high-molecular weight hyaluronic acid injections (Hyruan Plus Inj.) once weekly for 3 weeks in the treatment of patients with Grade I to III OA of the knee
Material and Methods
Study design
Phase III trial, Multi-center, Randomized, Parallel-group, Active-controlled, Double-blind
Primary Outcome measures
Change in weight-bearing pain (100mm-VAS) in 12 weeks after the final administration in comparison with baseline value
Safety Outcomes
- Adverse events
- Local reaction on the injection site
- Vital signs
- Laboratory tests
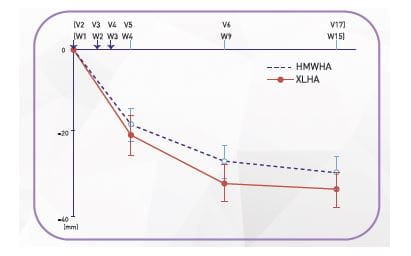
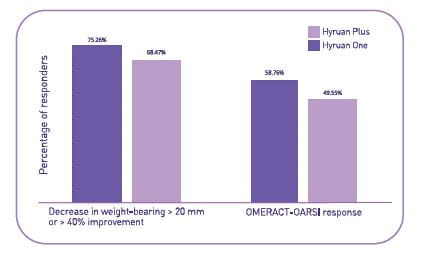
Results of Efficacy Analysis
Decrease in pain
- Significant reduction in weight bearing pain was observed at 1 week after the last injection and it was further evident at week 6 and week 12.
- The changes observed at 12 weeks after the last injection were still significant in both groups and the two-sided 95% CI for between-group difference was from −1.9 mm to 10.1 mm.
HMWHA, high molecular hyaluronate; XLHA, cross-linked hyaluronate; V, Visit; ; Error bars indicate 95% confidence interval of mean change
Responder rate (Per-protocol population)
Results of Safety Analysis
Number of patients experiencing common adverse Events (>1% of Population)
Conclusion: Hyruan One is an effective and safe treatment for Knee Osteoarthritis
Study 247
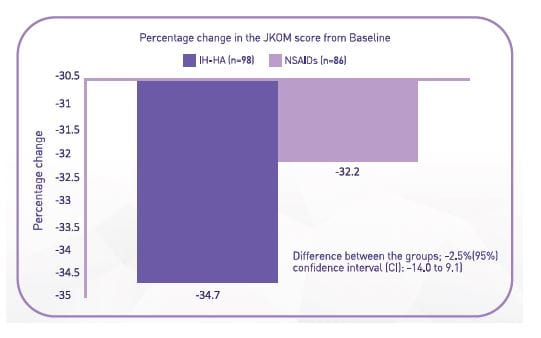
Comparison study of the efficacy and safety between IA-HA and NSAIDs for patients with knee OA
This study compared the efficacy and safety of early-phase IA-HA with NSAIDs in patients with knee OA with Kellgren-Lawrence (K/L) grade 1 to 3
- IA-HA was not inferior to NSAID.
- The frequency of both withdrawal and adverse events in the IA-HA group were significantly lower than those in the NSAID group (P=0.026 and 0.004, respectively).
|
Conclusion: The early efficacy of IA-HA is demonstrated to be not inferior to that of NSAIDs. The safety of the early phase of IA-HA is superior to that of NSAIDs for patients with knee OA. |
Study 336
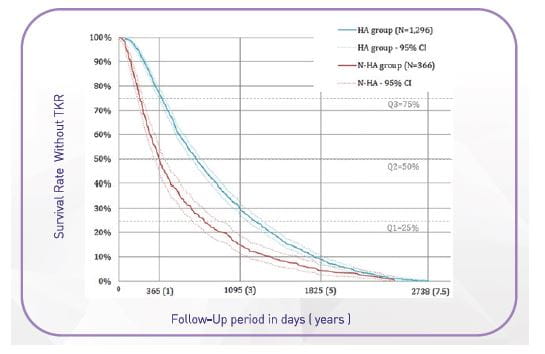
A study aimed to describe patients treated for knee osteoarthritis between 2006 and 2013 in France and compared the delay from diagnosis to total knee replacement between patients who received intra-articular hyaluronic acid.
A total of 14,782 patients were treated for knee osteoarthritis (67% women; mean age = 68 years). Among this population, 1,662 patients had total knee replacement (11.2%).
Approximately 7.5 years after diagnosis, patients who received HA had an additional time-to-TKR of 217 (±10) days (0.6 years/7.1 months) on average compared to patients who did not receive HA (p-value<0.0001).
Conclusion: Patients who received IA HA has an additional time-to-TKR as compared to patients who did not receive IA HA.
Study 443
Efficacy of 1 shot vs 3 shot vs 5 shot of Intra-Articular Hyaluronic acid injection
Hyruan One (60mg/3ml): 1 shot Intra articular Hyaluronic acid injection
Hyruan Plus (20mg/2ml): 3 shot Intra articular Hyaluronic acid injection
Hyruan (25mg/2.5ml): 5 shot Intra articular Hyaluronic acid injection
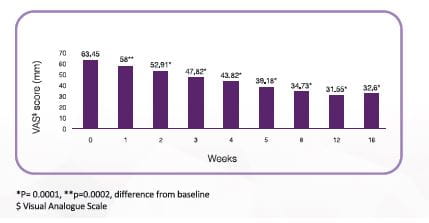
Five shots: Effect of Hyruan (2.5mL/week for 5 weeks) on pain in patients with grade I-III OA# (N=48)
Pain was significantly improved in 1 week and sustained till 16 weeks after administration of Hyruan Injection compared to before administration
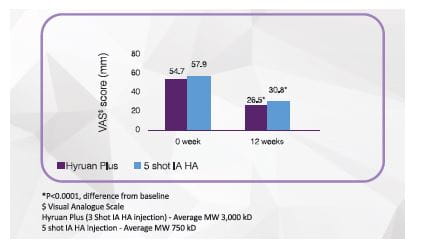
Three shot: Effect of Hyruan Plus vs 5 shot Intra-articular Hyaluronic Acid (IA HA) on pain in patients with grade I-III OA# (N=146)
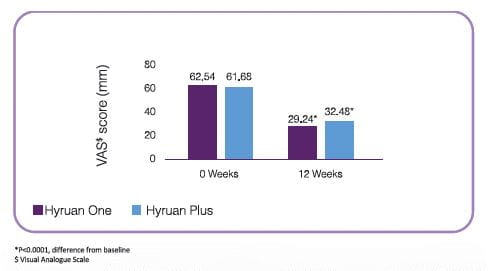
Single shot: Effect of Hyruan One (60mg/3mL) vs Hyruan Plus on pain in patients with grade I-III OA# (N=208)
Hyruan One and Hyruan Plus both reduces weight bearing pain in patients with Knee OA significantly after 12 weeks of administration compared to baseline
Hyruan One single shot injection is comparable to Hyruan Plus 3 shot injection in terms of efficacy with reduced number of injections.
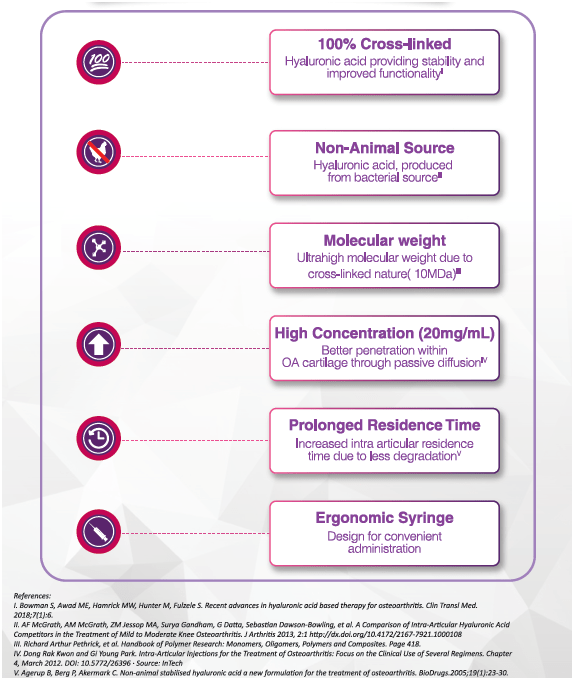
Unique Features and Benefits of Hyuran One
Offers comprehensive benefits in knee OA
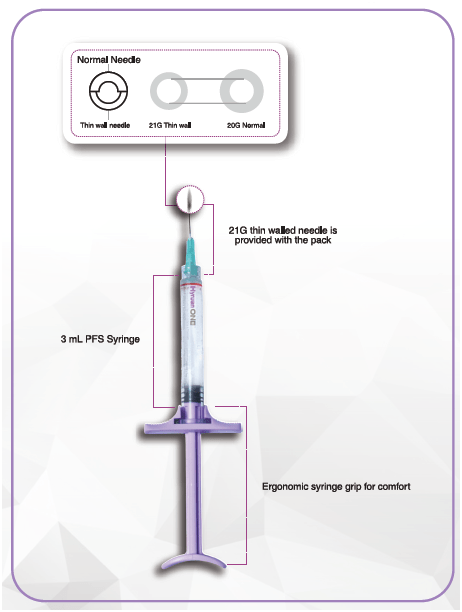
Syringe design for better convenience
Prescribing Information
For the use of a Registered Medical Practitioner or Hospital or a Laboratory Only.
Rx
Cross-linked Hyaluronic Acid Injection 20mg/ml
Hyruan ONE™
Naturally occurring hyaluronic acid acts in the joints as a shock absorber, protects cartilage and relieves pain associated with osteoarthritis. Hyruan ONE™ is a once-administered milestone visco supplement product which contains BDDE {l,4-butanediol diglycidyl ether)-crosslinked sodium hyaluronate as active ingredient and it is used to relieve pain of osteoarthritis of the knee.
Hyruan ONE TM is a clear, colorless and viscous gel containing 60 mg BDDE crosslinked sodium hyaluronate in a 3mL pre-filled syringe with rubber stoppers at both ends.
Contents
Each Hyruan ONE TM (volume of 3.0ml) contains:
Active ingredient: BDDE-crosslinked sodium hyaluronate gel - 3.0g
(BDDE·cross-linked sodium hyaluronate - 60mg)
Composition
Each mL contains BDDE·Cross-linked sodium Hyaluronic Acid .................... 20 mg
Indication
Osteoarthritis of the knee
Dose and administration
Hyruan ONE TM is a single injection, single dose preparation and should only be injected once per treatment course. The recommended dose is 3mL for knee joint.
Hyruan ONE TM should be administered by a medical professional.
Contraindications
Hyruan ONE™ should not be injected:
- To patients who are known to be sensitive to the product or ingredients of it.
- To patients with an infection or severe inflammation at joint cavity
- To patients with a disease or infection of skin near the injection site
Hyruan ONE TM should be injected with cautions:
- In patients with hypersensitivity to other substance
- In patients with liver disease or prior history of the liver disease
Adverse events
- Serious adverse events
Shock: Since symptoms of shock (frequency unknown) may occur, careful observation should be made. In case abnormalities are noticed administration should be discontinued and proper measures should be taken.
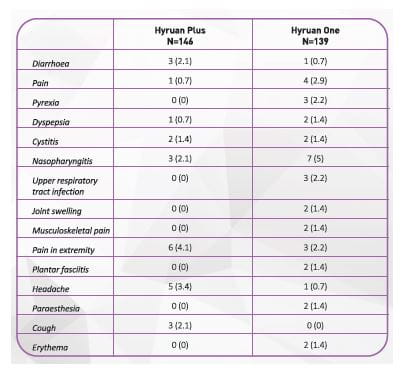
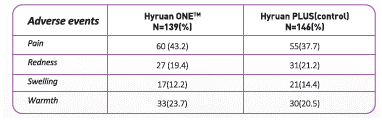
- In pivotal trial of Hyruan ONE™ in patients with the knee osteoarthritis (a total of 285 subjects), the occurrence rate of local reaction at injection site after injection into articular cavity was 48.9% (68 /139 subjects) in the study group and 49.3% (72/146 subjects) in the control group. The reported adverse events are shown in Table l. The serious adverse event were reported in the decreasing order of pain (7.2% of the study group, 6.2% of the control group), redness (erythema) (5.0% of the study group, 2.1% of the control group) and swelling (1.4% of the study group, 2.1% of the control group). Adverse events lasting over a week were pain 5.3%, redness 1.4%, swelling and warmth each accounting for 1.0%,yet all of them completely resolved in two weeks without any special treatment.
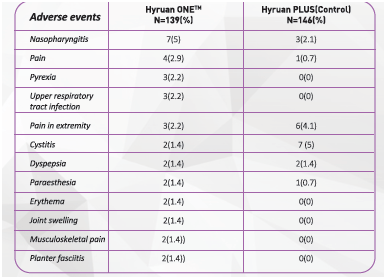
3) Of the patients with the knee osteoarthritis (285 subjects), the occurrence rate of the adverse events excluding ones occurred at the injection site is 34.5% (48/139, 73 cases) for the study group and 28.8% (42/146, 63 cases) for the control group. Most of them were mild to moderate.
Table 2 indicates the adverse events occurred in more than 1% of the study group
General Precautions
1) General Precautions
- Administration of Hyruan ONE™ to severely inflamed joints caused by deformative osteoarthritis can lead to exacerbation of the local inflammation symptoms. Thus Hyruan ONE™ is desired to be given after the removal of existing inflammation symptoms.
- Administration of Hyruan ONE™ may occasionally cause local pain or swelling, therefore patients should be
informed to avoid strenuous exercise or action leading to joint pain of knee up to 48 hours after
administration, and actions such as local relaxation should be guided after injection.
- Leakage of Hyruan ONE™ at an area other than articular cavity might cause pain; therefore, Hyruan ONE™ should be accurately injected into articular cavity.
2) Precautions in use
- Since Hyruan ONE™ is injected directly into the joints, administration should be performed under intact sterilization status.
- In case of retention of articular fluid, the fluid should be removed by puncturing prior to administration of Hyruan ONE™, if necessary.
- Intravascular injection, extra articular injection or injection in the synovial tissues should be avoided.
- It is desirable to administer the product using enclosed needles.
- Care should be taken with a disinfectant fourth grade ammonium salt such as benzalkonium chloride and chlorohexidine.
- Hyruan ONETM is intended for single use only. No re-sterilization or reuse is allowed. Do not use if
packaging or syringe is opened or damaged
- The injection site must be sterilized either by alcohol or other disinfecting solution to administration.
- Used syringe, needle and unused materials all need to be discarded after administration.
- In case Hyruan ONE™ is injected into both knees, a separate product should be used for each administration.
3) Precautions in handling
- Hyruan ONE™ should be kept away from children.
- Hyruan ONE™ should be kept in original packaging; storing Hyruan ONETM in a different packaging can cause misuse of product or decline the product quality.
Incompatibilities
Safety and efficacy of interactions between Hyruan ONETM and with other intraarticular injectables have not been established, therefore administration of Hyruan ONE™ with other intra-articular injectables is not recommended.
Use in specific populations
I) Pregnancy and nursing mothers
Safety and efficacy of Hyruan ONE™ in pregnant women and nursing mothers have not been established therefore administration of Hyruan ONE™ to pregnant women or nursing mothers is not recommended.
2) Paediatrics
The paediatric safety and efficacy of Hyruan ONE™ have not been established, therefore administration of Hyruan ONE™ to children is not recommended.
3) Geriatrics
Since physiological function of elderly patients has a declining tendency, administration should be made carefully. Although the number of patients over the age of 65 treated with Hyruan ONETM is not sufficient as 18.5% (521287), difference in the reaction incidence rate after administration of Hyruan ONETM between geriatric and non-geriatric group was not observed in the clinical trial.
Storage condition
Store between 2 - 8 °C in a hermetic container. Keep away from light. Keep out of sight and reach of children.
How supplied
One 3-0 mL pre-filled syringe and one needle (21 G)
Shelf life
24 months
References
1. Pal, C.P., et al., Epidemiology of knee osteoarthritis in India and related factors. Indian J Orthop, 2016. 50(5): p. 518-522.
2. Osteoarthritis, in Priority diseases and reasons for inclusion. Available at: https://www.who.int/medicines/areas/priority_medicines/Ch6_12Osteo.pdf?ua=1 Accessed on 12 Dec 2019.
3. Chandra Shekhar Azad., A.K.S., Poorti Pandey., Manish Singh., Pritee Chaudhary., Neelam Tia., Amit Rastogi and Indrajeet Singh Gambhir, Osteoarthritis in India: An epidemiological aspect. International Journal of Recent Scientific Research, 2017. 8(10): p. 20918-20922.
4. Haq, S.A. and F. Davatchi, Osteoarthritis of the knees in the COPCORD world. Int J Rheum Dis, 2011. 14(2): p. 122-9.
5.Chopra, A., et al., Prevalence of rheumatic diseases in a rural population in western India: a WHO-ILAR COPCORD Study. J Assoc Physicians India, 2001. 49: p. 240-6.
6.Palazzo, C., et al., Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med, 2016. 59(3): p. 134-138.
7.Jiang, L., et al., Body mass index and susceptibility to knee osteoarthritis: a systematic review and meta-analysis. Joint Bone Spine, 2012. 79(3): p. 291-7.
8.Hunter DJ, B.-Z.S., Osteoarthritis. Lancet, 2019.
9.Sokolove, J. and C.M. Lepus, Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis, 2013. 5(2): p. 77-94.
10.Glyn-Jones, S., et al., Osteoarthritis. Lancet, 2015. 386(9991): p. 376-87.
11.Troeberg, L. and H. Nagase, Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim Biophys Acta, 2012. 1824(1): p. 133-45.
12.Wojdasiewicz, P., L.A. Poniatowski, and D. Szukiewicz, The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm, 2014. 2014: p. 561459.
13.Yaftali, N.A. and K. Weber, Corticosteroids and Hyaluronic Acid Injections. Clin Sports Med, 2019. 38(1): p. 1-15.
14.Papakonstantinou, E., M. Roth, and G. Karakiulakis, Hyaluronic acid: A key molecule in skin aging. Dermatoendocrinol, 2012. 4(3): p. 253-8.
15.Altman, R.D., et al., The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord, 2015. 16: p. 321.
16.Hunter, D.J. and S. Bierma-Zeinstra, Osteoarthritis. Lancet, 2019. 393(10182): p. 1745-1759.
17.Fibel, K.H., H.J. Hillstrom, and B.C. Halpern, State-of-the-Art management of knee osteoarthritis. World J Clin Cases, 2015. 3(2): p. 89-101.
18.da Costa, B.R., et al., Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet, 2017. 390(10090): p. e21-e33.
19.Zeng, C., et al., Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: a systematic review and network meta-analysis of randomised controlled trials and observational studies. Br J Sports Med, 2018. 52(10): p. 642-650.
20.Jones, I.A., et al., Intra-articular treatment options for knee osteoarthritis. Nat Rev Rheumatol, 2019. 15(2): p. 77-90.
21.Martin, C.L. and J.A. Browne, Intra-articular Corticosteroid Injections for Symptomatic Knee Osteoarthritis: What the Orthopaedic Provider Needs to Know. J Am Acad Orthop Surg, 2019. 27(17): p. e758-e766.
22.Hochberg, M.C., et al., Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: a multicentre, randomised, double-blind, non-inferiority trial versus celecoxib. Ann Rheum Dis, 2016. 75(1): p. 37-44.
23.Cooper, C., et al., Use of Intraarticular Hyaluronic Acid in the Management of Knee Osteoarthritis in Clinical Practice. Arthritis Care Res (Hoboken), 2017. 69(9): p. 1287-1296.
24.Kopka, M., et al., Arthroscopy Association of Canada Position Statement on Intra-articular Injections for Knee Osteoarthritis. Orthop J Sports Med, 2019. 7(7): p. 2325967119860110.
25.Weber, M., et al., Predictors of Outcome After Primary Total Joint Replacement. J Arthroplasty, 2018. 33(2): p. 431-435.
26.Crawford, R.W. and D.W. Murray, Total hip replacement: indications for surgery and risk factors for failure. Ann Rheum Dis, 1997. 56(8): p. 455-7.
27.Ruth, L. Knee Replacement and Revision Surgeries on the Rise.
28.Bitton, R., The economic burden of osteoarthritis. Am J Manag Care, 2009. 15(8 Suppl): p. S230-5.
29.Dasa, V., S. Lim, and P. Heeckt, Real-World Evidence for Safety and Effectiveness of Repeated Courses of Hyaluronic Acid Injections on the Time to Knee Replacement Surgery. Am J Orthop (Belle Mead NJ), 2018. 47(7).
30.Henrotin, Y., et al., Consensus statement on viscosupplementation with hyaluronic acid for the management of osteoarthritis. Semin Arthritis Rheum, 2015. 45(2): p. 140-9.
31.Agerup, B., P. Berg, and C. Akermark, Non-animal stabilized hyaluronic acid: a new formulation for the treatment of osteoarthritis. BioDrugs, 2005. 19(1): p. 23-30.
32.Ghosh, P. and D. Guidolin, Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: are the effects molecular weight dependent? Semin Arthritis Rheum, 2002. 32(1): p. 10-37.
33.de Rezende, M.U. and G.C. de Campos, VISCOSUPPLEMENTATION. Rev Bras Ortop, 2012. 47(2): p. 160-4.
34.Watterson, J.R. and J.M. Esdaile, Viscosupplementation: therapeutic mechanisms and clinical potential in osteoarthritis of the knee. J Am Acad Orthop Surg, 2000. 8(5): p. 277-84.
35.Guidolin, D.D., et al., Morphological analysis of articular cartilage biopsies from a randomized, clinical study comparing the effects of 500-730 kDa sodium hyaluronate (Hyalgan) and methylprednisolone acetate on primary osteoarthritis of the knee. Osteoarthritis Cartilage, 2001. 9(4): p. 371-81.
36.Delbarre, A., et al., Do intra-articular hyaluronic acid injections delay total knee replacement in patients with osteoarthritis - A Cox model analysis. PLoS One, 2017. 12(11): p. e0187227.
37.Bruyere, O., et al., A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis-From evidence-based medicine to the real-life setting. Semin Arthritis Rheum, 2016. 45(4 Suppl): p. S3-11.
38.Moreland, L.W., Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Ther, 2003. 5(2): p. 54-67.
39.Altman, R.D., et al., Product Differences in Intra-articular Hyaluronic Acids for Osteoarthritis of the Knee. Am J Sports Med, 2016. 44(8): p. 2158-65.
40.Szilvia Berkóa, M.M., Magdolna Bodnárc, et al, Advantages of cross-linked versus linear hyaluronic acid for semisolid skin delivery systems. European Polymer Journal, 2013. 49(9): p. 2511-2517.
41.Bowman, S., et al., Recent advances in hyaluronic acid based therapy for osteoarthritis. Clin Transl Med, 2018. 7(1): p. 6.
42.De Boulle, K., et al., A review of the metabolism of 1,4-butanediol diglycidyl ether-crosslinked hyaluronic acid dermal fillers. Dermatol Surg, 2013. 39(12): p. 1758-66.
43.Data on file.
44.Jackson, D.W. and T.M. Simon, Intra-articular distribution and residence time of Hylan A and B: a study in the goat knee. Osteoarthritis Cartilage, 2006. 14(12): p. 1248-57.
45.Leighton, R., et al., Systematic clinical evidence review of NASHA (Durolane hyaluronic acid) for the treatment of knee osteoarthritis. Open Access Rheumatol, 2018. 10: p. 43-54.
46.Ha, C.W., et al., Efficacy and safety of single injection of cross-linked sodium hyaluronate vs. three injections of high molecular weight sodium hyaluronate for osteoarthritis of the knee: a double-blind, randomized, multi-center, non-inferiority study. BMC Musculoskelet Disord, 2017. 18(1): p. 223.
47.Ishijima, M., et al., Intra-articular hyaluronic acid injection versus oral non-steroidal anti-inflammatory drug for the treatment of knee osteoarthritis: a multi-center, randomized, open-label, non-inferiority trial. Arthritis Res Ther, 2014. 16(1): p. R18.