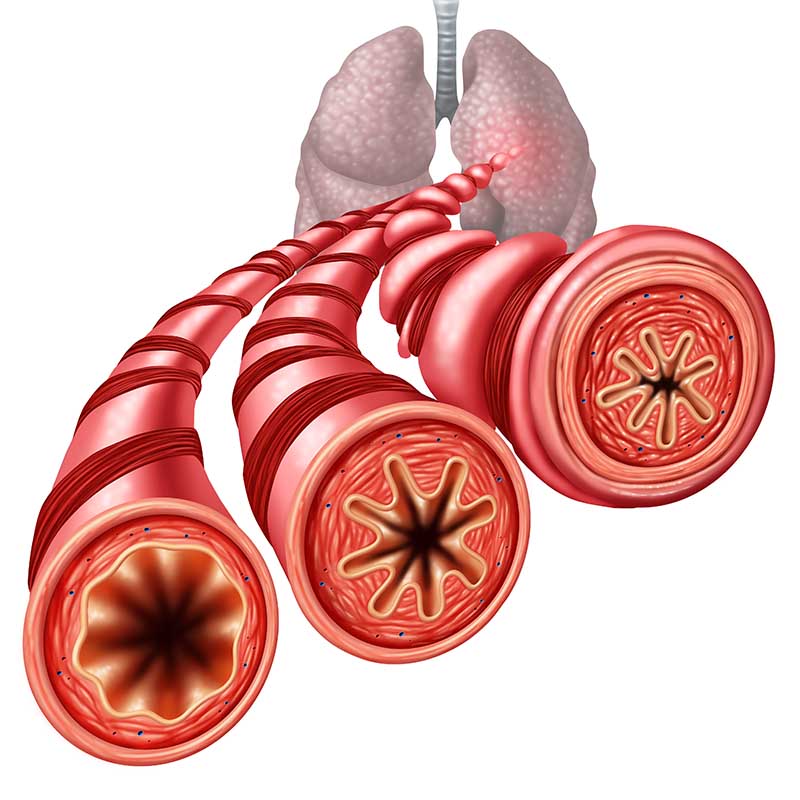
Nasal polyposis is a common clinical condition that, despite differing hypotheses on its cause, remains a poorly understood disease. Histologically, polyps consist of respiratory epithelium covering very oedematous stroma infiltrated by a number of inflammatory cells such as eosinophils, mast cells and lymphocytes. For most patients the management of nasal polyps comprises a combination of medical and surgical therapies, but it is important to consider possible aetiological factors and association with systemic diseases such as asthma and intolerance to aspirin, cystic fibrosis, primary ciliary dyskinesia and immune deficiency, and to exclude the possibility of inverted papilloma, allergic fungal sinusitis or an underlying neoplasm in all cases before treatment.
Intranasal Steroids in Nasal Polyposis
Introduction
Clinical Definition of Nasal Polyps
Inflammation of the Nose and the Paranasal Sinuses Characterized by Two or More Symptoms
- Blockage/ congestion
- Discharge: anterior/ post nasal drip
- Facial pain/ pressure
- Reduction or loss of smell
Endoscopic Signs
- Polyps
- Mucopurulent discharge from middle meatus
- Oedema/mucosal obstruction primarily in middle meatus
CT Changes
- Mucosal changes within osteomeatal complex and/or sinuses
Clinical Definition of Nasal Polyps is Based on
- Nasal symptomatology
- Endoscopic signs and/or
- CT changes
Objectives of Treatment
In discussing and comparing different types of therapy, it is useful to define the objectives for management.
These are
- The elimination of nasal polyps or a considerable reduction of their size
- Re-establishing an open nasal airway and nasal breathing
- Improvement and/or restoration of the sense of smell, and
- Prevention of recurrence of nasal polyps, although realistically a delay of recurrence is anticipated rather than the elimination of all sinus pathology.
Classification and Staging of Nasal Polyps
A system for classifying and staging nasal polyposis should be universally adopted to allow for accurate comparison of different therapies and of the same therapies in different series. At an international workshop on nasal polyposis in Davos, Switzerland, in March 1996, the system described by Lund and Mackay in 1993 and accepted at the International Conference on Sinus Disease: Terminology, Staging and Therapy was updated. This system grades polyps (table I), allows for the inclusion of systemic diagnoses that may influence outcome, and scores the extent of disease on computed tomography (CT) scan (table II).
Score 0, 1 or 2 points for each region:
0 point = No mucosal thickening
1 point = Mucosal thickening
2 points = Sinus opacification
Diagnosis of Nasal Polyposis
Subjective Assessment is Based on Symptoms
- Nasal blockage, congestion or stuffiness
- Nasal discharge or postnasal drip, often mucopurulent
- Facial pain or pressure, headache
- Reduction/ loss of smell
- Dysphonia
- Cough
- Drowsiness
- Malaise
Examination
- Anterior rhinoscopy
- Endoscopy
- Nasal Cytology (To indicate the aetiology of the disease)
- Biopsy (To exclude neoplasia and other severe conditions)
- Microbiology (for confirmation of pathogens and their response to therapy)
Imaging
- Plain sinus X-rays
- CT scanning
- MRI (for investigation of severe conditions such as neoplasia)
Management of Nasal Polyps
Evidence based management scheme for ENT specialists:
Mechanisms of Corticosteroid Effect
Topical corticosteroids are the medical treatment of choice in nasal polyposis. The introduction of modern corticosteroid sprays in the 1970s was followed by a series of studies that shed light upon the effect of corticosteroids on various aspects of the inflammatory reaction in the airway mucosa. Corticosteroids have a multifactorial effect initiated by their binding to a specific cytoplasmic glucocorticoid receptor.
Within hours of administration, the drug-receptor complex modifies gene transcription and induces a change of protein synthesis within the cell. Although the individual response of patients is variable, this change of protein synthesis is responsible for the clinical effect which becomes apparent within a matter of days in nasal polyposis. Within hours of administration, the steroid-receptor complex modifies gene transcription and induces a change of protein synthesis within the cell that inhibits formation of inflammatory mediators.
T Cells and Cytokines
There is accumulating evidence that the inflammatory reaction in nasal polyposis is at least in part driven by T cells and their humoral products, the cytokines. Kanai et al have shown that corticosteroids significantly alter T-cell kinetics by reducing the total number of lymphocytes, their activation and cytokine production.
Mast Cells
The role of mast cells in nasal polyposis is unclear but prolonged corticosteroid treatment in the airways reduces the number of mast cells in asthma and allergic rhinitis, and may well be of importance in nasal polyposis. Stem cell factor, a mast cell growth and survival factor produced by fibroblasts, appears to be suppressed by corticosteroid treatment.
Eosinophils
Topical corticosteroids reduce the influx and total number of eosinophils in polyp tissue. Corticosteroids may inhibit the influx of these cells by suppressing the production of Regulated upon Activation, Normal T cell Expressed and Secreted (RANTES), a cytokine released by fibroblasts probably involved in eosinophil recruitment. Recent research demonstrates that apoptosis (programmed cell death) in inflammatory cells is an important factor in the resolution of inflammation, and apoptosis is induced in eosinophils in cell culture with corticosteroids.
Topical Corticosteroid Treatment
Once the diagnosis has been established intranasal corticosteroids are the best documented treatment for nasal polyps and there are several placebo-controlled studies that have shown a significant clinical effect. However, nasal corticosteroids alone do not solve all problems for patients with nasal polyps. This may be because the patient is genuinely unresponsive to corticosteroids as may occur in cystic fibrosis, primary ciliary dyskinesia and other diseases characterised by a predominantly neutrophilic rather than eosinophilic infiltration. Also, the disease can be temporarily unresponsive because of inadequate delivery of the medication in a very blocked nose with grade 3 polyps or with purulent infection. In this clinical situation, systemic corticosteroid therapy or surgery may be necessary in the first instance followed by subsequent topical therapy.
Topical corticosteroids are of use in the primary treatment of nasal polyps, once the diagnosis has been established, and in the maintenance of any therapeutic improvement.
Commonly Used Intranasal Steroids
- Mometasone furoate
- Fluticasone propionate
- Budesonide
- Flunisolide
- Triamcinolone Acetonide
- Beclomethasone
Lipid Solubility of Intranasal Corticosteroids
Highly lipophilic agents have a greater degree and faster rate of absorption into nasal mucosa.
Enhanced ability to reach GR due to longer retention time in nasal tissue.
Rank order of lipid solubility (highest to lowest):
- Mometasone
- Fluticasone
- Beclomethasone
- Budesonide
- Triamcinolone
- Flunisolide
Clinical Studies
The efficacy of intranasal corticosteroid sprays such as beclomethasone dipropionate (beclomethasone), budesonide and fluticasone propionate in reducing polyp size and rhinitis symptoms has been demonstrated in several placebo-controlled trials. Flunisolide, budesonide and beclomethasone dipropionate have been shown to delay the recurrence of polyps after surgery.Table below summarises these medical trials.
Medical Trials Showing the Efficacy of Intranasal Corticosteroids
A review of the evidence from controlled trials reveals that all studies of topical corticosteroids have shown an effect on nasal blockage but less improvement in olfaction. Polyps may shrink or may no longer be visible on anterior rhinoscopy in approximately 50% of patients.
Chalton et al found disappearance of polyps in 9 of 15 patients using betamethasone drops compared with 2 of 15 in the placebo group.
A more recent placebo-controlled study by Lund et al compared beclomethasone dipropionate with fluticasone and showed that both sprays were equally effective in treating the symptoms of severe nasal polyps. Nasal blockage was significantly decreased in both active groups compared with the placebo group, the polyp score was significantly decreased in the fluticasone group, the peak nasal inspiratory flow rate was greater in the fluticasone group and the nasal cavity volume was significantly increased in both groups. There was some evidence that the group treated with fluticasone responded faster than the group receiving beclomethasone. This is in accordance with the results reported by Holmberg et al in which a significant reduction in polyp size was seen in patients after 14 weeks' treatment with fluticasone or with beclomethasone, but with evidence that fluticasone has a faster onset of action (Fig 3).
A study by Albertien et al demonstrated the efficacy of Fluticasone propionate nasal drops in reducing the need for surgery in patients with polyposis and chronic rhinosinusitis. There was a reduction of nasal symptoms and also a decrease in polyp volume and an improvement in CT scores.
Lildholdt et al found a decrease in the mean polyp size in 52% of budesonide treated patients compared with 21% in the placebo group at the end of 3 months. Similarly, nasal peak flow could be shown to improve significantly over a 4-month period in patients using budesonide compared with those using placebo. Johansen et al found a clear reduction in polyp score and an improvement in nasal peak flow during treatment with budesonide.
Mometasone furoate has also demonstrated a reduction in polyp size and an improvement in the nasal symptom scores in a study by Bloom M et al (Fig 4). Mometasone also improved the sense of smell.
Both Fluticasone and Mometasone have shown a reduction in polyp size and an improvement in nasal symptom scores.
Many patients find the loss of sense of smell and appreciation of flavour one of the most disturbing symptoms of nasal polyposis but only a few studies have looked at the effect of intranasal corticosteroids on these symptoms in an objective way. El-Naggar et al studied the possible benefit of beclomethasone dipropionate on olfactory function in 29 patients postnasal polypectomy. The University of Pennsylvania Smell Identification Test (UP-SIT) was performed and they found no statistically significant difference in the scores between treated and untreated nostrils.
Intranasal corticosteroids probably have their greatest role in maintenance therapy. Intranasal steroids have been shown to delay the recurrence of polyps after surgery. During a 30-month postpolypectomy observation period of 20 patients treated with beclomethasone dipropionate, Karlsson and Rundcrantz found it necessary to perform only 4 repeat polypectomies compared with 14 in the untreated control group. Beclomethasone dipropionate was also found to be effective in the reduction of symptomatic recurrence in 54% of actively treated patients in a study by Virolainen and Puhakka. Although the reappearance of nasal polyps can be prevented in some cases, it would appear that in most patients the use of topical corticosteroids will reduce the frequency of relapses.
Conclusion
The treatment plan of nasal polyposis should be tailored for each individual patient but most will require a combination of medical and surgical therapy to deal with clinical recurrences that characterize this condition. It has been shown that topical corticosteroid treatment can reduce polyp size and the majority of associated nasal symptoms. The initial management of patients with small or medium sized polyps (grades 1 and 2) may be with topical corticosteroids. When polyps are large it may be difficult to instil the medication, and surgery or short term systemic corticosteroids may be required. Treatment after polypectomy significantly reduces the number of recurrences, which is especially valuable in patients who have previously been subjected to frequent polypectomies.
Thus, topical corticosteroid sprays are of considerable value for long term maintenance but with the knowledge that from time to time the other strategies may be necessary, and this of course will be influenced by other factors such as the presence of asthma and/or aspirin sensitivity. In theory 'long term therapy' could imply 'life-long' therapy but in practice, most patients will eventually stop treatment if they remain asymptomatic. Trials looking at post-operative topical corticosteroid therapy for 5 years or longer are being conducted but results are not as yet available. The safety profile of modern topical corticosteroids as evidenced by studies in rhinitis would not raise any significant concerns, particularly when balanced against the reduction in morbidity and need for surgical intervention.
Treatment plan of nasal polyposis should be tailored for each individual patient but most will require a combination of medical and surgical therapy to deal with clinical recurrences.
Topical corticosteroid treatment can reduce polyp size and the majority of associated nasal symptoms.References
1 .Drugs 2001; 61(5): 573-578
2. Allergy 2005; 60: 583-601
3. J Allergy Clin Immunol 1999; 104, S: 144-149
4. U.S. Pharmacist July 2002: 81-90
5. J Allergy Clin Immunol 2005; 115: 1017-1023