Kelfer
Data Sheet
Composition
Description
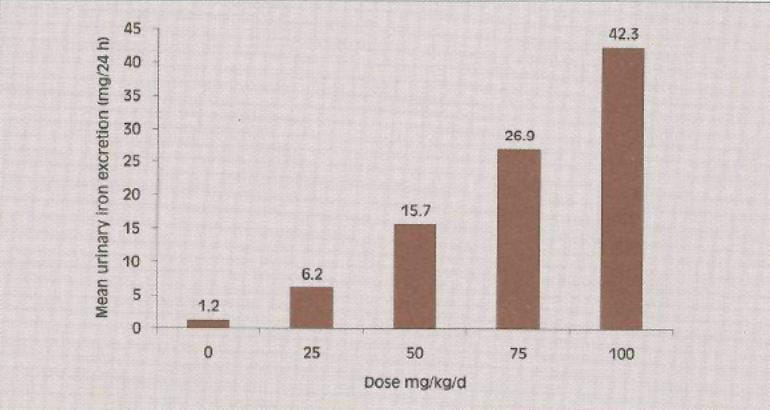
Fig. 1: Dose related urinary iron excretion in deferiprone-treated patients
Pharmacokinetics
Absorption
Deferiprone is rapidly absorbed from the upper part of the gastrointestinal tract. Peak serum concentration is reported to occur 45 to 60 minutes following a single dose in fasted patients. This may be extended to 2 hours in fed patients. Following a dose of 25 mg/kg, lower peak serum concentrations have been detected in patients in the fed state (85 ?mol/l) than in the fasting state (126 ?mol/l), although there was no decrease in the amount of deferiprone absorbed when it was given with food.
Biotransformation
Deferiprone is metabolized predominantly to a glucuronide conjugate. This metabolite lacks iron-binding capability due to inactivation of the 3-hydroxy group of deferiprone. Peak serum concentrations of the glucuronide occur 2 to 3 hours after administration of deferiprone.
Elimination
In humans, deferiprone is eliminated mainly via the kidneys; 75% to 90% of the ingested dose is reported as being recovered in the urine in the first 24 hours, in the form of free deferiprone, the glucuronide metabolite and the iron-deferiprone complex. A variable amount of elimination via the faeces has been reported. The elimination half-life in most patients is 2 to 3 hours.
Clinical Efficacy
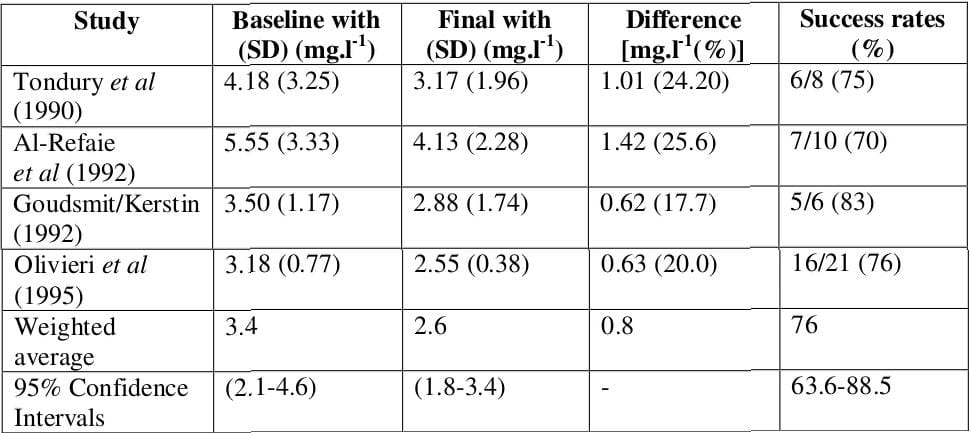
Meta-Analysis
Table 1: Meta-analysis of the clinical effectiveness of deferiprone
Indian Study
Combination of Desferrioxamine and Deferiprone
Table 2: Combination of deferiprone and DFO (n = 28, 16 ± 8.6 months)
Indications
Dosage and Administration
In adults and children, the optimum dose of Kelfer Capsules to achieve a negative iron balance is 75 mg/kg/day to be administered in two to four divided doses. In some patients a lesser dose of 50 mg/kg/day may be adequate while in others the dose may be increased to 100 mg/kg/day.
A total daily dose above 100 mg/kg body weight is not recommended because of potentially increased risk of adverse reactions.
The effect of deferiprone in decreasing the body iron is directly influenced by the dose and the degree of iron overload. After starting deferiprone therapy, it is recommended that serum ferritin concentrations, or other indicators of body iron load, be monitored every 2 to 3 months to assess the long-term effectiveness of the chelation regimen in controlling the body iron load. Dose adjustments should be tailored to the individual patient's response and therapeutic goals (maintenance or reduction of body iron burden). Interruption of therapy with deferiprone should be considered if serum ferritin measurements fall below 500 ?g/l.
Contraindications
Warnings and Precautions
Neutropenia/Agranulocytosis
Deferiprone has been shown to cause neutropenia, including agranulocytosis. The patient's neutrophil count should be monitored every 3 to 4 weeks.
In the Event of Neutropenia
Instruct the patient to immediately discontinue deferiprone and all other medicinal products with a potential to cause neutropenia. The patient should be advised to limit contact with other individuals in order to reduce the risk of infection. Obtain a complete blood cell (CBC) count, with a white blood cell (WBC) count, corrected for the presence of nucleated red blood cells, a neutrophil count, and a platelet count immediately upon diagnosing the event and then repeat daily. It is recommended that following recovery from neutropenia, weekly CBC, WBC, neutrophil and platelet counts continue to be obtained for 3 consecutive weeks, to ensure that the patient recovers fully. Should any evidence of infection develop concurrently with the neutropenia, the appropriate cultures and diagnostic procedures should be performed and an appropriate therapeutic regimen instituted.
In the Event of Severe Neutropenia or Agranulocytosis
Follow the guidelines above and administer appropriate therapy such as granulocyte colony-stimulating factor, beginning the same day that the event is identified; administer daily until the condition resolves. Provide protective isolation and, if clinically indicated, admit the patient to the hospital. Limited information is available regarding rechallenge. Therefore, in the event of neutropenia, rechallenge is not recommended. In the event of agranulocytosis, rechallenge is contraindicated.
Drug Interactions
Due to the unknown mechanism of deferiprone-induced neutropenia, patients must not take medicinal products known to be associated with neutropenia or those that can cause agranulocytosis. Interactions between deferiprone and other medicinal products have not been reported. However, since deferiprone binds to metallic cations, the potential exists for interactions between deferiprone and trivalent cation-dependent medicinal products such as aluminium-based antacids. Therefore, it is not recommended to concomitantly ingest aluminium-based antacids and deferiprone. The safety of concurrent use of deferiprone and vitamin C has not been formally studied. Based on the reported adverse interaction that can occur between deferoxamine and vitamin C, caution should be used when administering deferiprone and vitamin C concurrently
Plasma Zn2+ Concentration
Monitoring of plasma Zn2+ concentration, and supplementation in case of a deficiency, is recommended. HIV-positive or Other Immune-compromised Patients: Given that deferiprone can be associated with neutropenia and agranulocytosis, therapy in immune-compromised patients should not be initiated unless potential benefits outweigh potential risks. No data are available on the use of deferiprone in HIV-positive or in other immune-compromised patients
In Impaired Renal and Hepatic Function
Since deferiprone is excreted mainly via the kidneys, Kelfer Capsules should be used with caution in this category of patients. Renal and hepatic function should be monitored in this patient population during deferiprone therapy. If there is a persistent increase in serum alanine aminotransferase (ALT), interruption of deferiprone therapy should be considered. In thalassaemia patients, there is an association between liver fibrosis and iron overload and/or hepatitis C. Special care must be taken to ensure that iron chelation in patients with hepatitis C is optimal. In these patients, careful monitoring of liver histology is recommended
Discolouration of Urine
Patients should be informed that their urine may show a reddish/brown discolouration due to the excretion of the iron-deferiprone complex.
Chronic Overdose and Neurological Disorders
Neurological disorders have been observed in children treated with 2.5 to 3 times the recommended dose for several years. Prescribers are reminded that the use of doses above 100 mg/kg/day are not recommended.
Pregnancy
Deferiprone is not recommended for use in pregnant women. Lactation: It is not known whether deferiprone is secreted into breast milk. In such cases, benefits to the mother must be weighed against the risks to the child.
Paediatric Use
There is no clinical experience of Kelfer Capsules in children below 2 years of age. Therefore, its use is not recommended.
Side Effects
Gastrointestinal Tract
Adverse effects involving the gastrointestinal tract such as anorexia, nausea, vomiting, gastric discomfort and altered taste have occurred commonly. In most of these patients, the effects have disappeared on continuation of therapy.
Arthropathy
Joint pains have been reported in 10 to 30% of patients receiving deferiprone. These usually involve knees, ankles, elbows, hip and the lower back. In a few patients, the small joints of the hands and feet have been involved. In some patients, swelling of the joints with effusion has been seen. The exact mechanism of this arthropathy is not known, but it appears to be by a mechanism other than immunological. Joint pains have been seen more commonly in patients with a higher serum ferritin and those on higher dose of deferiprone. These may be related to deposition of iron in the joints (due to the chelation process), leading to an inflammatory reaction in the joints. If joint pains occur, it may be necessary to stop the drug for a short while or reduce the dosage. The joint pains usually disappear and the drug may be restarted at lower doses. Concomitant use of nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibrupofen or diclofenac may be used to control the pain. In a few patients, however, joint pains may recur on restarting the drug and, thus, such patients may be unable to tolerate the drug (also see Patient Monitoring).
Agranulocytosis and Neutropenia
Deferiprone has caused agranulocytosis and neutropenia in a few patients (also see Patient Monitoring).
Elevated Liver Enzymes
Increased levels of serum liver enzymes have been reported in some patients taking deferiprone. In the majority of these patients, the increase was asymptomatic and transient, and returned to baseline without discontinuation or decreasing the dose of deferiprone.
Progression of Fibrosis
Some patients experienced progression of fibrosis associated with an increase in iron overload or hepatitis C.
Neurological Disorders
Neurological disorders (such as cerebellar symptoms, diplopia, lateral nystagmus, psychomotor slowdown, hand movements and axial hypotonia) have been observed in children who had been voluntarily prescribed more than 2.5 times the maximum recommended dose of 100 mg/kg/day for several years. The neurological disorders progressively regressed after deferiprone discontinuation.
Zinc Depletion
Zinc depletion has been reported on prolonged treatment, leading to dermatopathy in 1% of patients. This can be easily corrected by giving zinc supplements.
Patient Monitoring
The minimum monitoring that is essential for deferiprone therapy is as follows:
a. Haemoglobin, total and differential white cell counts and platelet counts at 3- to 4-weekly intervals or whenever clinically indicated.
b. Serum ferritin at 3- to 4-monthly intervals
Note
If the total white cell count drops to less than 3,000/cmm or absolute neutrophil count (ANC) falls to less than 1,000/cmm or platelet count falls to less than 1,00,000/cmm, the drug should be discontinued. If the patient develops fever, sore throat or flu-like symptoms, the drug should be stopped and a complete blood count done immediately. If neutropenia is seen, appropriate therapy should be instituted. The patient may have to be hospitalized till the counts come back to normal. In such patients, deferiprone should not be restarted. In case the patient develops severe joint pain, swelling or difficulty in squatting/walking and no relief is obtained by administering ibuprofen/diclofenac or any other suitable NSAID, the therapy should be discontinued. The drug should not be restarted if joint pains recur.
Presentation
Kelfer-250 Capsules
Container of 50 capsules
Kelfer-500 Capsules
Container of 50 capsules
References
2. Drug Safety 1997; 17:407-21
3. 9th International Conference on Oral Chelation in the treatment of thalassaemia and other diseases. March 25-28, 1999 Hamburg, Germany. P19
4. Indian J Pediatr 1993; 60:509-16
5. Drugs 1999; 58:553-78