Lument 80
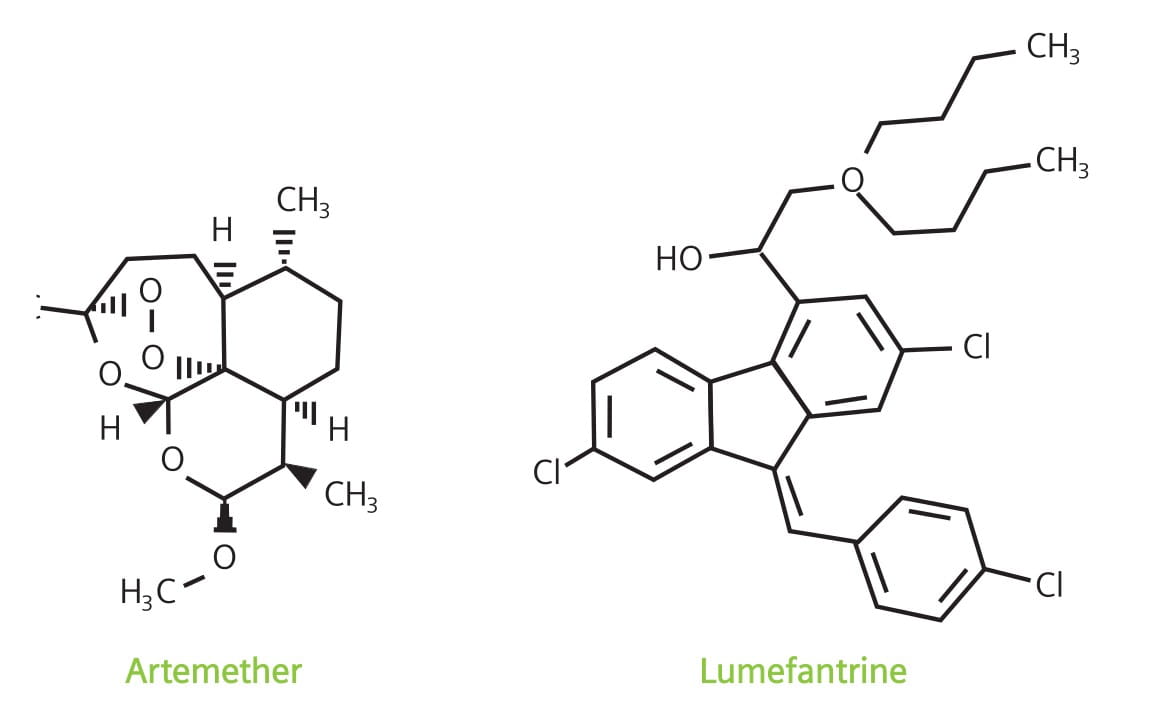
Artemether 80 mg / Lumefantrine 480 mg
Fast Result ,Better Compliance
LUMET 80 is a fixed dose, artemisinin-based combination containing artemether, an artemisinin derivative, and lumefantrine, a synthetic antimalarial drug.
Each LUMET 80 tablet contains artemether 80 mg and lumefantrine 480 mg.
Lumefantrine has a much longer elimination half-life than artemether, and is associated with a low recrudescence rate, but has a slower onset of action. However, when used together, the complementary properties of artemether, with its fast onset of action, and lumefantrine, with its long duration of action and high cure rate, result in a highly effective combination. 9
- Highly effective against uncomplicated malaria caused by P. falciparum in areas of multi-drug resistance.
- Eliminates parasites and symptoms significantly faster than most current antimalarials.
- Gametocytocidal, thus reduces transmission and prevents spread of resistant parasites.
- Achieves high cure rates and prevents recrudescence.
- Well tolerated, particularly when compared to most established antimalarials.
- Fixed-dose combination treatment and simple dosage regimen, therefore, high compliance.
Artemether acts rapidly with a half-life of 1 to 3 hours, whereas lumefantrine has a half-life of 3 to 6 days and is responsible for preventing recurrent parasitemia.
Artemether and lumefantrine have different modes of action and act at different points in the parasite life cycle. Artemether interferes with the parasite transport proteins, disrupts the parasite mitochondrial function, inhibits angiogenesis and modulates the host immune function. Lumefantrine is an aryl-amino alcohol that prevents detoxification of heme, such that toxic heme and free radicals induce parasite death.4
Artemether and lumefantrine differ in their rates of absorption and elimination. Artemether is rapidly absorbed, reaching peak plasma concentrations within 2 hours post-dose. It is metabolized rapidly by cytochrome P450 (CYP) 2B6, CYP3A4 and, possibly, CYP2A610 to dihydroartemisinin (DHA), which, in turn, is converted to inactive metabolites, primarily by glucoronidation via UGT1A1, 1A8/9 and 2B7. The metabolite, DHA, reaches peak plasma concentrations within 2 to 3 hours post-dose. Both artemether and DHA offer potent antimalarial properties, causing a significant reduction in the asexual parasite mass of approximately 10,000-fold per reproductive cycle, along with prompt resolution of symptoms.4
Lumefantrine is absorbed and cleared more slowly, acting to eliminate residual parasites that may remain after artemether and DHA have been cleared from the body and, thus, prevents recrudescence. Lumefantrine is highly lipophilic, thus absorption is enhanced with a fatty meal; its absorption occurs 2 hours after intake, reaching peak plasma concentrations after 3 to 4 hours, with an elimination half-life of 4 to 10 days.4
Food enhances the absorption of both artemether and lumefantrine although this effect is more apparent for lumefantrine. Administration of artemether/lumefantrine with a high-fatmeal increases the bioavailability of both artemether and lumefantrine by 2-fold and 16-fold, respectively.4
The effect of food on artemether/lumefantrine absorption is of concern because patients with malaria usually have anorexia, vomiting and low food intake. It is important to counsel patients to take artemether/lumefantrine with food.4
Artemether/lumefantrine (A/L) is a viable antimalarial combination treatment, because in addition to its efficacy, safety and tolerance profile, it is available as a fixed-dose formulation, increasing the likelihood of patient compliance with the drug regimen.
The hallmark of efficacy of an antimalarial is its ability to eliminate the malaria parasite from a patient's blood and other tissues, thereby enabling the disappearance of symptoms, and a cure. A variety of studies have evaluated the efficacy of A/L in terms of speed and degree of parasite elimination, and these have shown:
- High parasitological cure rates
- Fast parasite elimination
- Prompt reduction in fever
- Effective gametocyte clearance
- Effectiveness in multi-drug resistant areas
Adequate Clinical and Parasitological Response (ACPR): ACPR is defined as the absence of parasitaemia on day 14, irrespective of axillary temperature, and without previously meeting any of the criteria of ETF, LCF or LPF.
Cure Rate: Cure rate is usually defined as the 28-day cure rate, i.e., the proportion of patients with clearance of parasites within 7 days, and without subsequent return of the original infection during the 28-day period following the first dose.
Early Treatment Failure (ETF): ETF is defined as the development of danger signs or severe malaria on days 1, 2, or 3 in the presence of parasitaemia on day 2 higher than day 0 count, irrespective of axillary temperature, or parasitaemia on day 3 with axillary temperature of 37.5°C, or parasitaemia on day 3, which is >25% of day 0 count, irrespective of axillary temperature.
Endemic: A disease that exists permanently in a particular region or population.
Epidemic: An outbreak of disease that attacks many people at about the same time and may spread through one or several communities.
Evaluable Patients: Patients whose response to a treatment can be measured because enough information has been collected.
Fever Clearance Time (FCT): Time from the first dose to the time when body temperature falls to normal and remains so for at least 48 hours.
Late Clinical Failure (LCF): LCF is defined as the development of danger signs or severe malaria and/or axillary temperature of 37.5°C on any day from day 4 to day 14 in the presence of parasitaemia, without previously meeting any of the criteria of ETF.
Late Parasitological Failure (LPF): LPF is defined as the presence of parasitaemia on day 14 and axillary temperature <37.5°C, without previously meeting any of the criteria of ETF or LCF.
Parasite Clearance Time (PCT):PCT is the time needed to clear asexual parasite forms from the blood (i.e., where parasite numbers fall below the limit of detection in a thick blood smear and remain undetectable for at least 48 hours).
Recrudescence: The occurrence of parasites surviving in red blood cells after a brief check (e.g., after failed or incomplete drug treatment) and causing the reappearance of the disease.
Reinfection: A condition caused by a totally new infection, and not related to the survival of the parasite within the body.
A/L is a Viable Option for the Treatment of Uncomplicated Falciparum Malaria in India11
Aim: To assess the efficacy of A/L in India.
Study Site: Highly malaria endemic areas of India (Kamrup district, Assam, in northeastern India and Keonjhar district, Orissa, in the eastern part of India).
Participants: 124 patients (Assam=53, Orissa=71) with uncomplicated falciparum malaria (between 2 and 55 years of age).
Method: A/L (20 mg/120 mg) six-dose regimen (0, 8, 24, 36, 48 and 60 hours) was administered, according to weight, twice daily for 3 days, as follows:
- One tablet (10-14 kg), two tablets (15-24 kg), three tablets (25-34 kg), or four tablets (≥35 kg) per dose.
All the patients were followed for 28 days.
Results: 122 patients completed the study.
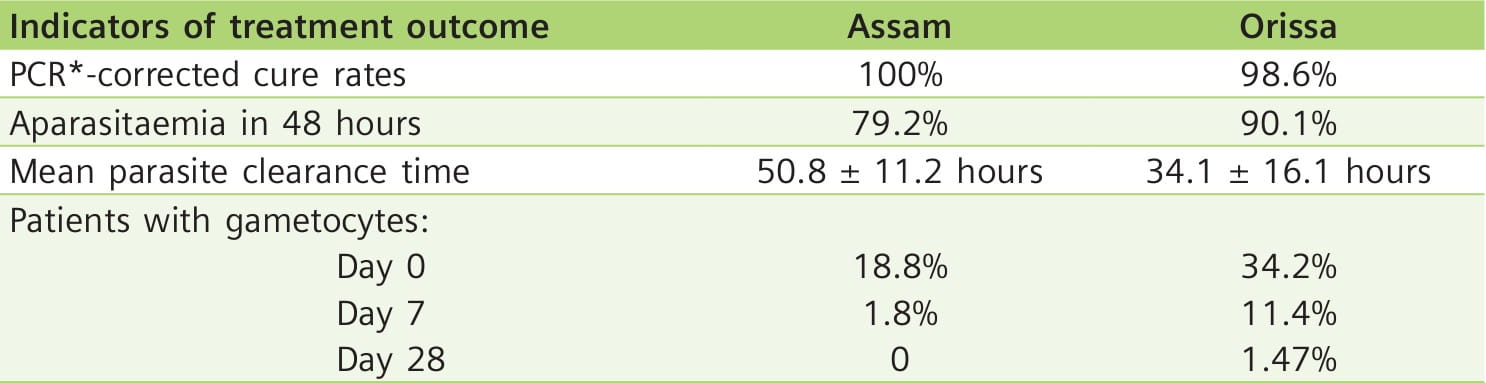
The only treatment failure case on day 7 was a malnourished 7-year-old child, weighing 11 kg, from Orissa.
The drug was well-tolerated with no adverse events.
Key Points
- A/L is a safe and effective drug for the treatment of acute uncomplicated falciparum malaria in India.
- Treatment failure can occur due to incomplete absorption of the drug as is suspected in one case of failure on day 7 in the study.
A/L and as + MQ are Equally Effective in Treating Uncomplicated Multi-Drug Resistant Falciparum Malaria12
Aim: To monitor and compare the efficacy of a 3-day course of A/L and AS + MQ.
Study Site: Mae Sot, Thailand, an area of multi-drug resistant P. falciparum infection.
Participants: 490 patients with uncomplicated falciparum malaria.
Method: Patients were randomly allocated to receive A/L or AS + MQ and followed for 42 days.
- Patients allocated to A/L, received the fixed-dose combination tablets (20 mg/120 mg) at 0, 8, 24, 36, 48, and 60 hours. The number of tablets were given according to body weight. The minimum dosage for patients weighing less than 15 kg was 1 tablet per dose; patients between 15 and 24 kg received 2 tablets; those between 25 and 34 kg received 3 tablets; and patients 35 kg and above were treated with 4 tablets per dose.
- Patients allocated to the AS + MQ group received AS, 4 mg/kg once daily for 3 days, plus MQ, 15 mg/kg on day 1 and 10 mg/kg on day 2.
Results: 484 patients (242 in each group) were included in the final evaluation. Of these, 6 patients (3 in each group) were excluded because they did not meet the WHO criteria.
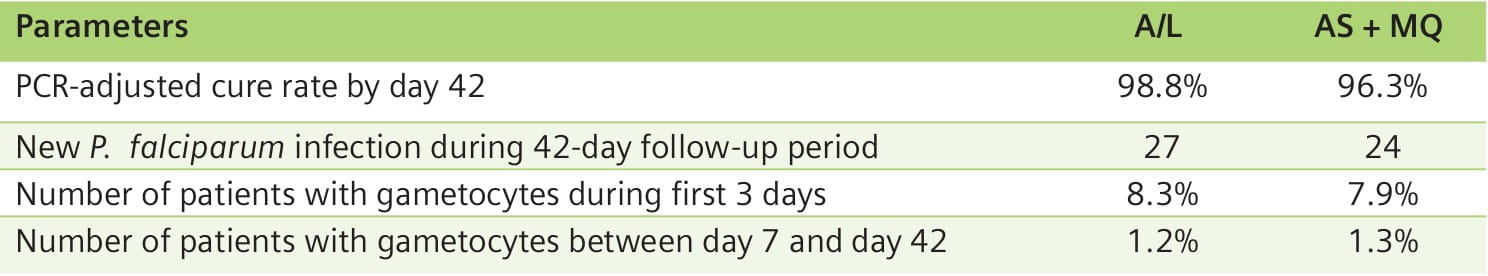
All patients had rapid initial clinical and parasitological responses. In both groups, the PCR- adjusted cure rates by day 42 were high: 98.8% for A/L and 96.3% for AS + MQ.
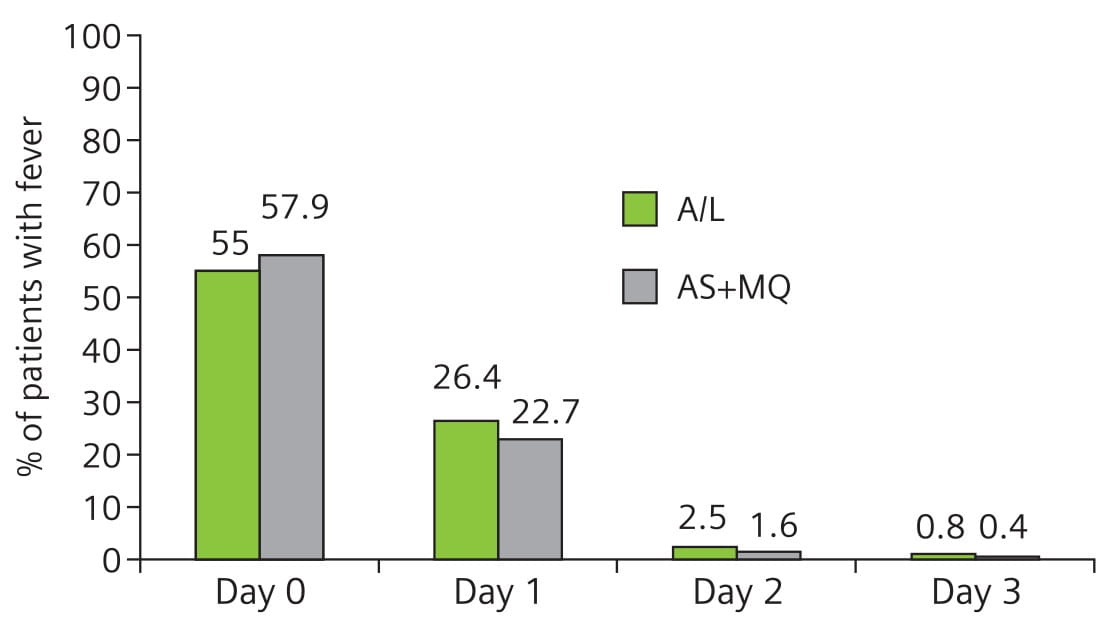
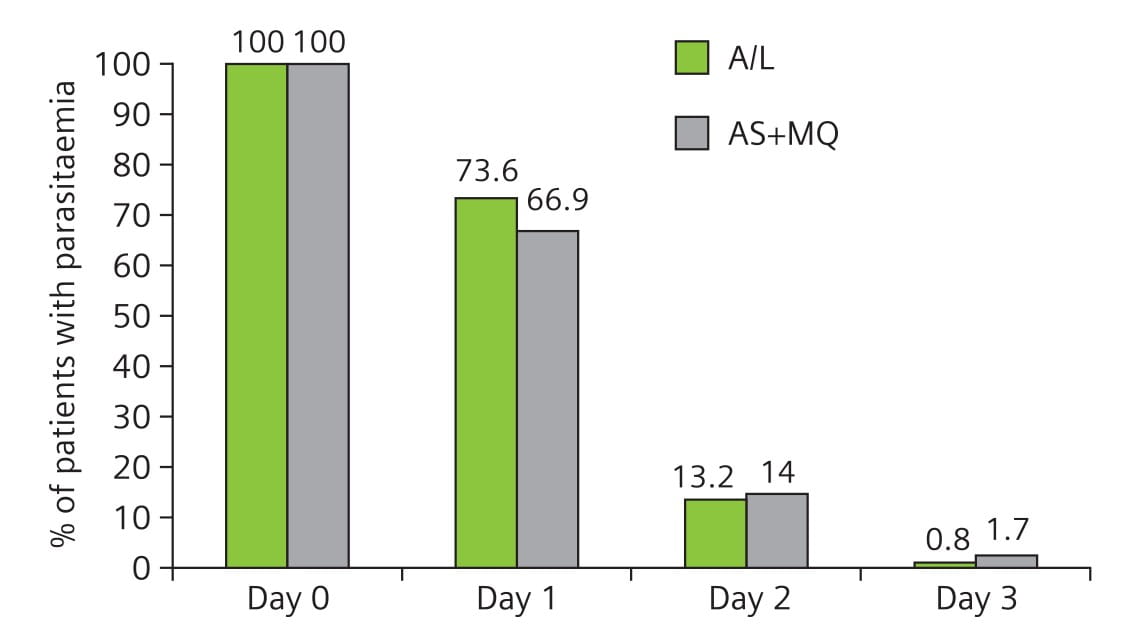
Except for 3 patients (2 in A/L and 1 in AS + MQ), all the others had normal temperature on day 3. There was no difference in FCTs between the two treatment groups (Fig. 2).
PCT was short and most patients cleared their parasitaemia by day 2 (Fig. 3).
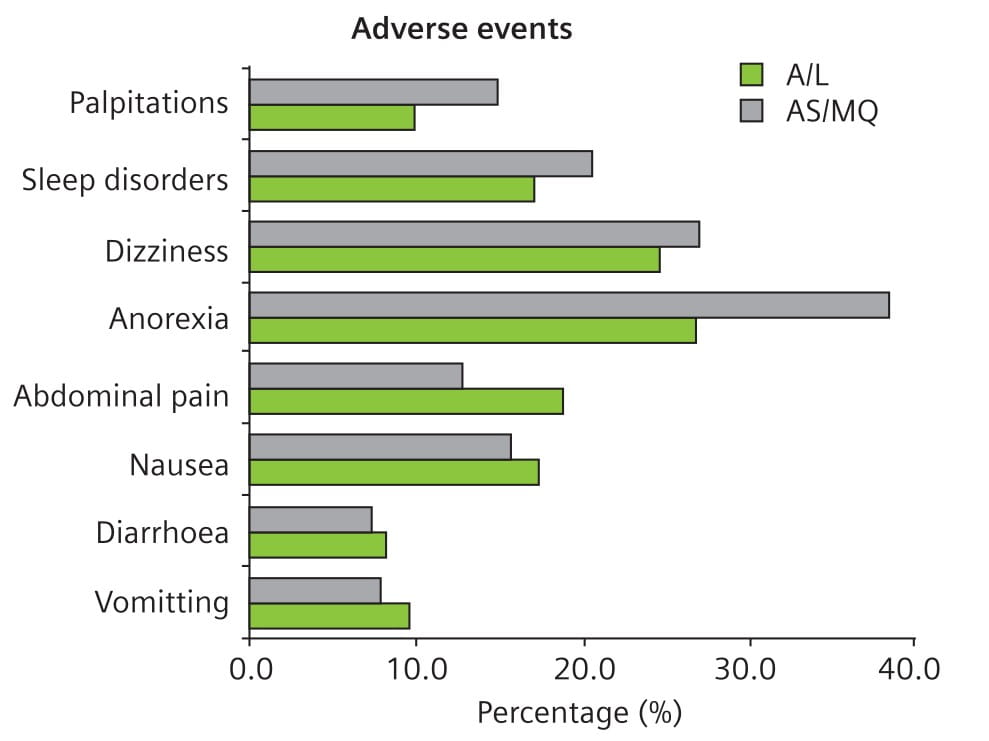
Both regimens were very well tolerated, with no serious adverse events observed attributable to either combination. Overall, there were less adverse events in the A/L group compared to the AS + MQ group, though the differences were not statistically significant (Fig. 4).
- Both A/L and AS + MQ were highly effective and resulted in equivalent therapeutic responses in the treatment of highly drug-resistant falciparum malaria.
- There were less side effects in the A/L group compared to the AS + MQ group.
Acts Highly Effective in Treating Uncomplicated Falciparum Malaria13
Aim: To compare the efficacy of four antimalarial combinations (A/L, AS+AQ, AS+MQ, and AQ+SP).
Study Site: The study was conducted in five health districts in Senegal. The transmission of malaria is moderate in these zones.
Participants: 955 patients (aged 1 to 75 years) with uncomplicated falciparum malaria.
Method: The patients were randomly assigned to receive one of the following treatments:
- A/L: Two dosages were administered.
- Four doses of A/L (n=140)
- Adults: Four doses of four tablets repeated after 8, 24 and 48 hours.
- Children weighing 5-15 kg: One tablet repeated after 8, 24 and 48 hours.
- Six doses of A/L (n=149)
- Six doses consisting of two daily doses for 3 days.
- AS+AQ (n=360)
- 4 mg/kg/day AS plus 10 mg/kg/day AQ, for 3 days.
- AS+MQ (n=145)
- Adults: 200 mg/day AS plus 250 mg/day MQ, for 3 days.
- Children: 100 mg/day AS plus 125 mg/day MQ, for 3 days.
- AQ+SP (n=161)
- 10 mg/kg/day AQ for 3 days, and ½ tablet per 10 kg of SP as a single dose only on the first day.
Patients were followed for 42 days.
Results: All the drug combinations demonstrated good efficacy. On day 28, all the combinations resulted in an excellent clinical and parasitological response rate of 100% after correction for PCR results, except for the four-dose A/L regimen (96.4%). Follow-up of approximately 10% of each treatment group on day 42 demonstrated an efficacy of 100% (Table 3). All the combinations were well-tolerated.
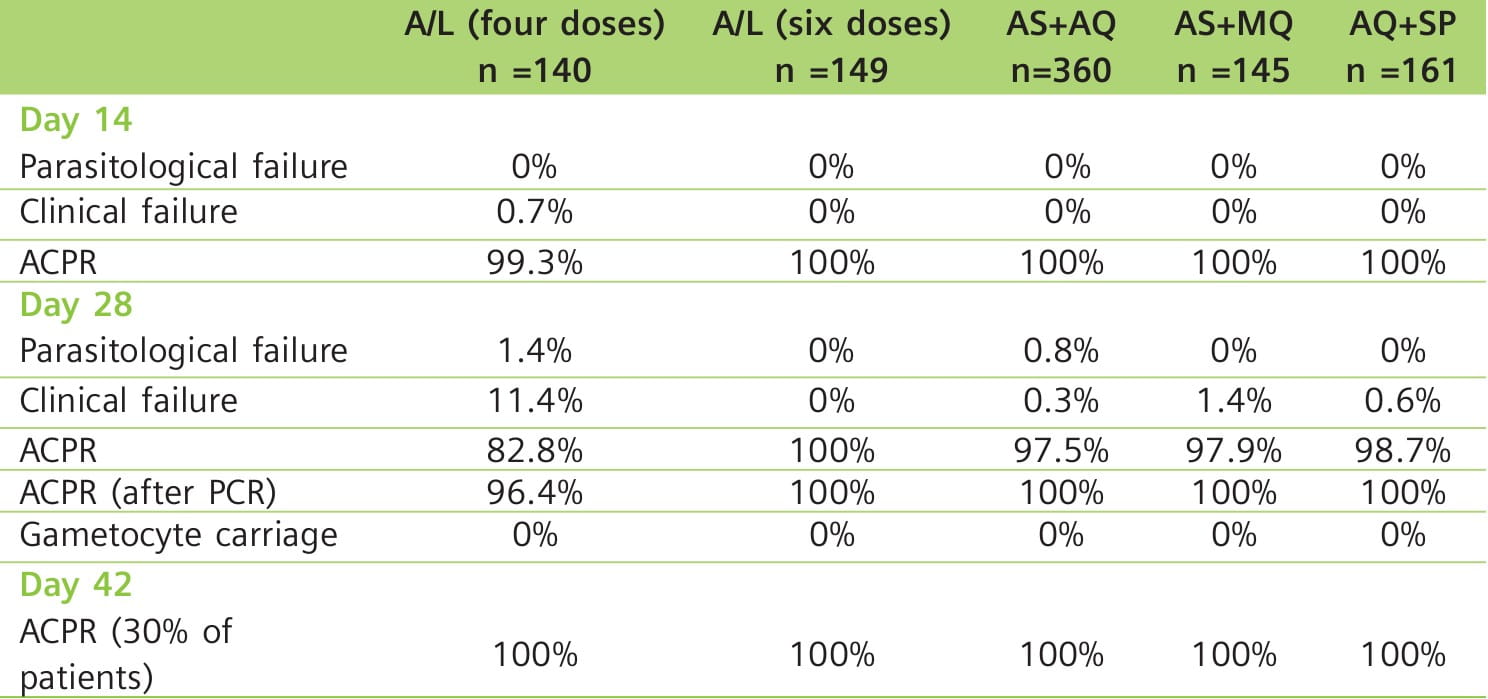
Fig. 5 shows that the parasitic clearance on days 1 and 2 was slower with AQ+SP compared with combinations containing an artemisinin derivative.
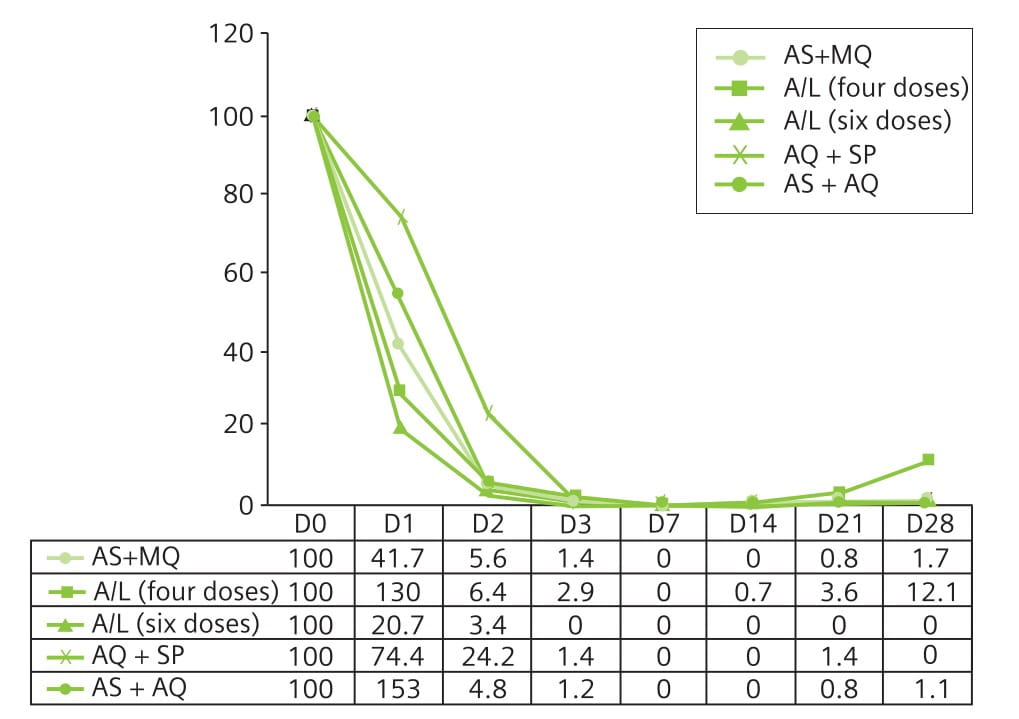
When gametocytaemia was investigated, a reduction in the gametocyte carriage was observed from day 3 for combinations containing an artemisinin derivative (Table 4). By days 21 and 28, no patient had detectable gametocytes.
Key Points
- All the tested antimalarial combinations demonstrated good clinical and parasitological efficacy. Only A/L given as four doses was associated with a failure rate of 3.6%
A/L More Efficacious and Convenient Than Quinine (Q) + Doxycycline (D)14
Aim: To compare the efficacy of A/L with that of Q + D in the treatment of uncomplicated falciparum malaria.
Study Site: West Amazon region of Brazil, which has a high level of resistance to a number of antimalarial agents.
Participants: 59 patients (≥16 years) with uncomplicated falciparum malaria.
Method: Patients were randomly assigned to receive A/L (n=28) or Q + D (n=31).
- Patients in the A/L (20 mg/120 mg) group received an initial dose of 4 tablets, followed by 4 tablets at 8, 24, 36, 48, and 60 hours.
- Patients in the Q + D group received Q (500 mg) every 8 hours for 3 days, and D (100 mg) every 12 hours for 5 days.
Results: 3 patients did not complete the study. In the A/L group, 1 patient withdrew consent because of personal problems and 2 patients in the Q + D group withdrew because of an adverse event (vomiting) and treatment ineffectiveness, respectively.
Patients in the A/L group were parasite-free on day 3, while those taking Q + D were parasite- free on day 6.

- A/L was better than Q + D with regard to simplicity of drug administration and patient convenience. In this study, A/L therapy required taking a single product twice a day for 3 days, amounting to six doses. The Q + D regimen necessitated taking 19 medication doses at various intervals.
- A/L therapy cleared parasitaemia faster than Q + D therapy.
Several current antimalarials have a relatively poor tolerability profile. Some may cause severe and even potentially lethal adverse events. It is important, therefore, that antimalarial treatments not only demonstrate high efficacy, but also have a safety profile that is at least equivalent, if not superior, to other drugs. The combination of artemether and lumefantrine meets these requirements.
Aim: A randomized comparison of the safety and tolerability of three oral antimalarial combinations: CQ + SP versus AS + MQ versus A/L.
Study Site: Savannakhet Province, Laos.
Participants: 330 patients (aged ≥ 1 year) with uncomplicated falciparum malaria.
Method: Patients randomly received one of the following regimens:
- CQ, 10 mg base/kg of body weight, followed by 10 mg base/kg after 24 hours, followed by 5 mg base/kg at 48 hours, plus SP, 25 mg/1.25 mg/kg on day 1, or
- AS, 4 mg/kg/day for 3 days (days 0-2), plus MQ, 15 mg/kg on day 1 and 10 mg/kg on day 2, or
- A/L, 20 mg/120 mg, respectively, per tablet, administered as one dose, by weight, at 12- hour intervals for 3 days [dosages were 1 tablet (<15 kg), 2 tablets (15-24 kg), 3 tablets (25-34 kg), and 4 tablets (≥ 35 kg)].
Patients were followed for 42 days.
Results: In almost all efficacy parameters, AS + MQ and A/L had a superior response than CQ + SP. An evaluation of safety and tolerability showed that A/L had the least side effects and was the best tolerated regimen (Table 5).
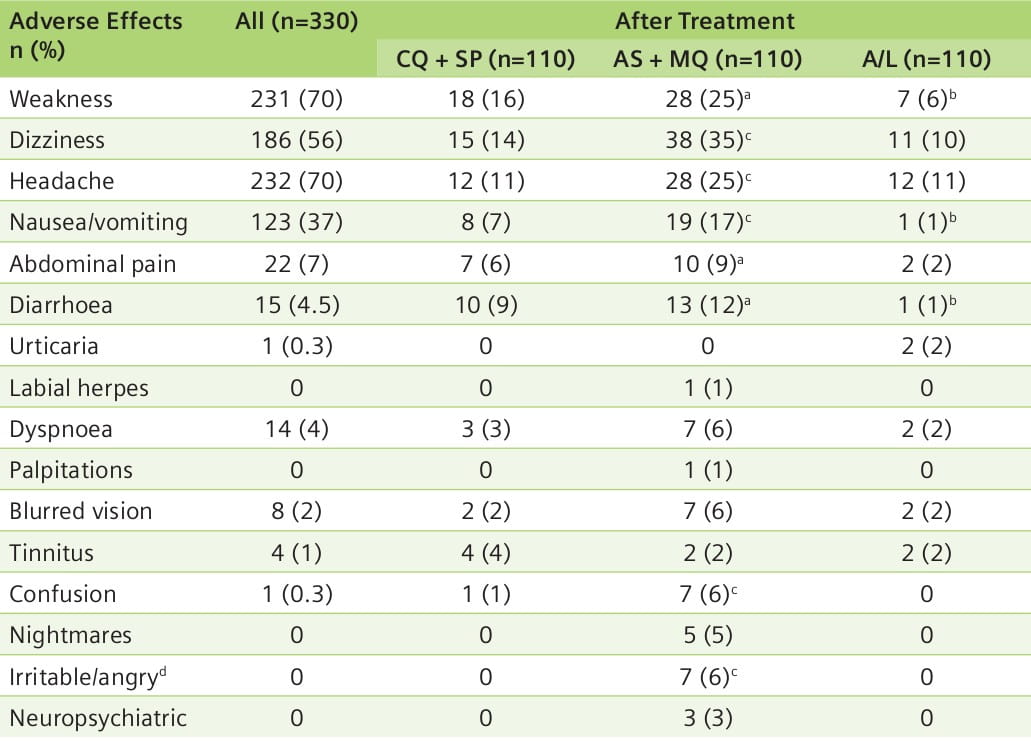
- AS + MQ and A/L were more effective treatments for malaria in southern Laos than CQ + SP.
- AS + MQ therapy was associated with significantly more serious, although transient, adverse effects as compared to A/L therapy.
Aim: To compare the cardiac effects of single oral doses of A/L and halofantrine in healthy males.
Participants: 14 healthy Caucasian males, aged 24-49 years.
Method: Single oral doses of 500 mg halofantrine or 80 mg/480 mg A/L were given to healthy males and the effects on the QTc interval were compared in a randomized, double-blind, crossover study. The aim was to achieve plasma concentrations similar to those observed in malaria patients after repeated dosing. A 6-week washout period was kept between the two treatments.
Electrocardiograms (ECGs) were recorded from 48 hours before dosing until 48 hours thereafter. The maximum QTc interval [QTc = QT/square root (RR)] was compared before and after treatment, and between treatments, fitting a general linear model. Drug plasma concentrations were determined concomitantly.
Results: 13 participants completed both study periods. After halofantrine, all participants showed an increase in the QTc interval (Fig. 6); the mean maximum increase was 28 minutes. The length of the QTc interval was positively correlated to halofantrine exposure. The QTc interval remained unchanged after A/L (Fig. 6). The difference between treatments was statistically significant (Fig. 7).
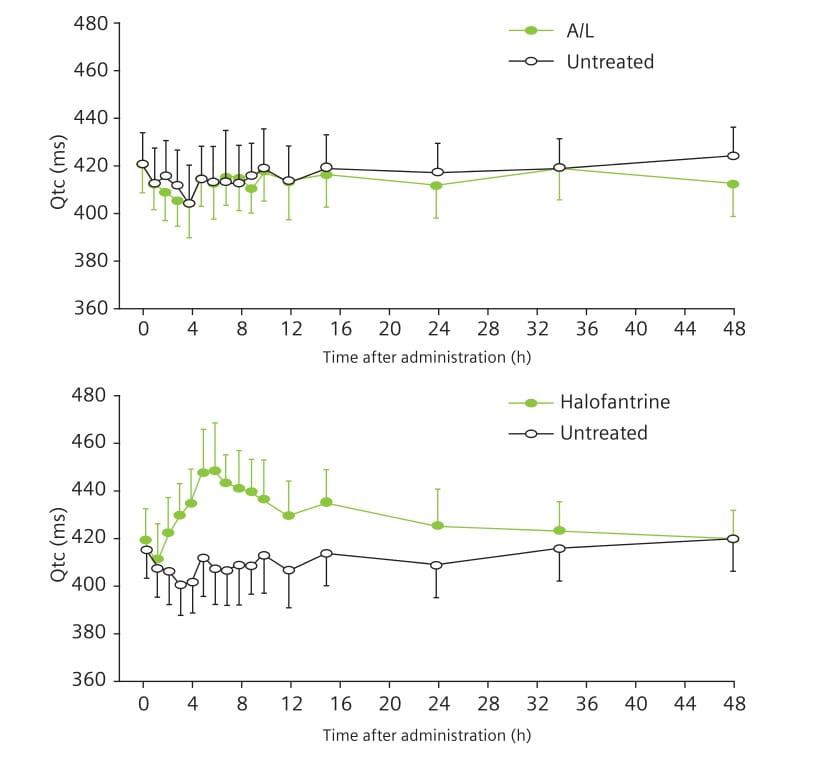
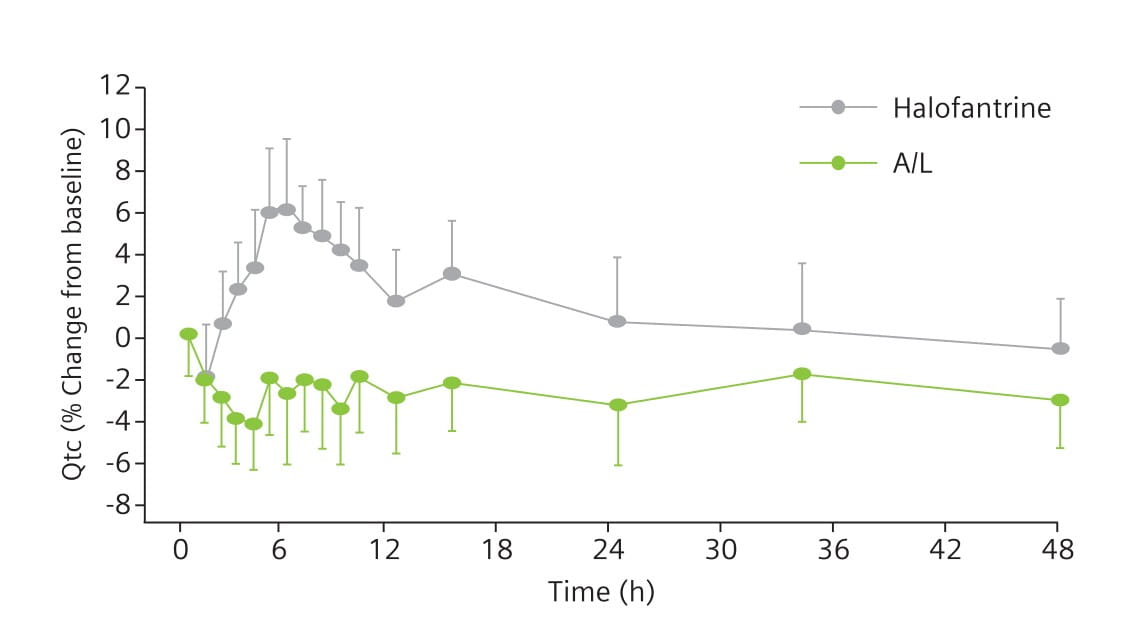
Key Points
Halofantrine caused a significant, exposure-dependent increase in the QTc interval. No such effect was seen with A/L.
Aim: To compare the ototoxicity, tolerability and efficacy of A/L with that of quinine sulphate (Q) and atovaquone/proguanil (AP) in the treatment of uncomplicated falciparum malaria.
Study Site: Jimma, southwest of Addis Ababa, Ethiopia.
Participants: 97 patients (>5 years of age) with uncomplicated falciparum malaria.
Method: Patients were assigned to one of the following treatment groups by stratified random sampling:
- A/L: 20 mg of artemether and 120 mg of lumefantrine (children 5-14 kg body weight [bwt.]) or 40 mg/240 mg (children 15-24 kg bwt.) or 60 mg/360 mg (children 25-34 kg bwt.) or 80 mg/480 mg (adults and children ≥35 kg bwt.), at hours 0, 8, 24, 36, 48 and 60 (six doses).
- Q: 10 mg/kg (children) or 600 mg (adults and children >50 kg bwt.), according to about 8 mg/kg Q base, three times daily for 7 days (twenty-one doses).
- AP: 20 mg/8 mg/kg (children <40 kg bwt.) or 1000 mg/400 mg (adults and children >40 kg bwt.) per day for 3 days (three doses).
Patients were followed for 90 days. Comprehensive audiovestibular testing by pure tone audiometry (PTA), transitory-evoked (TE) and distortion product (DP) otoacoustic emissions (OAE) and brain stem-evoked response audiometry (BERA) was done before enrollment and after 7, 28 and 90 days.
Results: 13 patients (7 in the Q group, 4 in the AP group and 2 in the A/L group) were not available for the day 90 examinations.
Clinical and parasitological efficacy
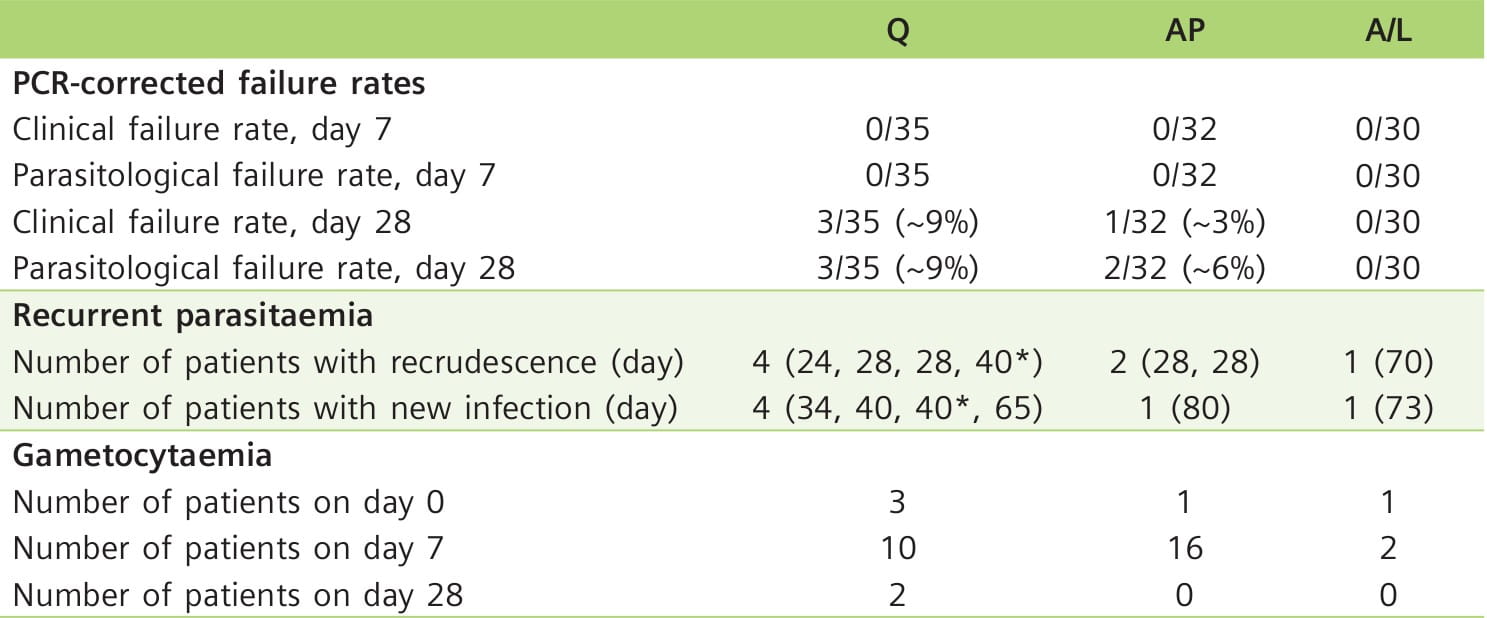
Table 6 shows the efficacy of the three treatment groups.
Tolerability and ototoxicity assessment
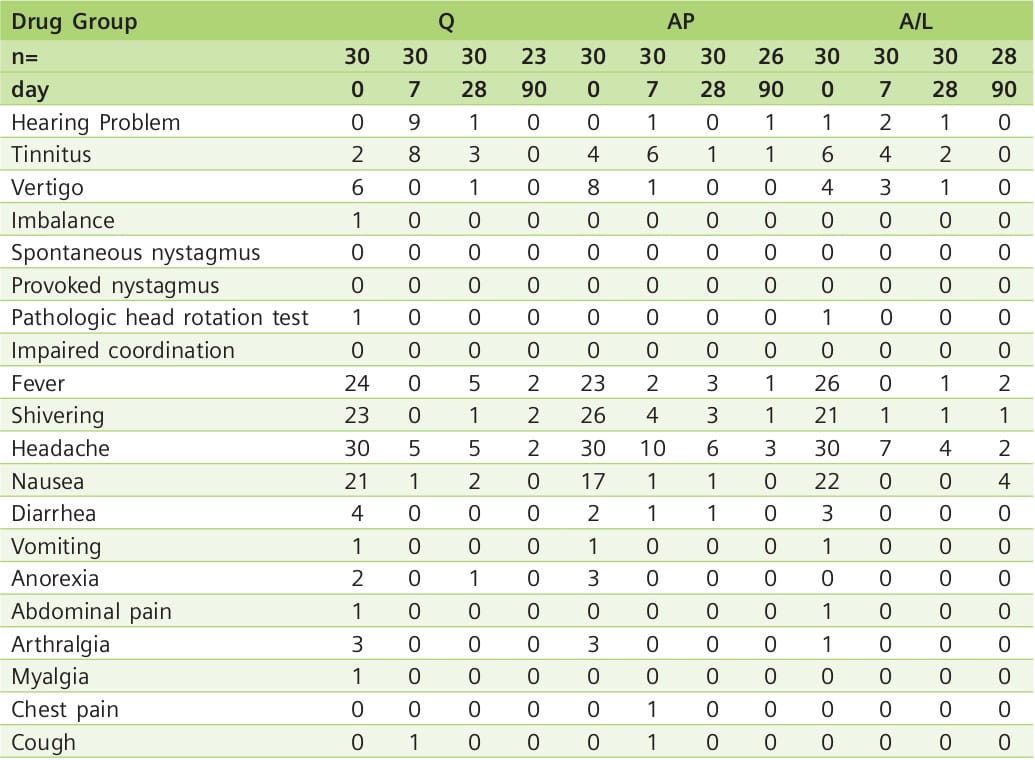
No vomiting occurred after the ingestion of the antimalarial drugs, and no serious adverse events were reported during treatment and follow-up. Most symptoms present at the time of diagnosis resolved by day 7 (Table 7). However, hearing problems and tinnitus were more common on day 7, with 9 of 30 patients complaining of hearing problems in the Q group. In 7 of these patients, audiometry and OAE testing confirmed significant hearing loss. Patients reporting subjective hearing impairment in the A/L group did not have abnormal hearing test results. In the AP group, only the reported hearing loss by 1 patient on day 90 corresponded to significantly impaired audiometry and OAE results; in this patient, malaria re-infection was diagnosed.
PTA and OAE
PTA and DP-OAE levels revealed transient significant cochlear hearing loss in patients treated with Q, but not in those treated with A/L or AP. TE-OAE could be elicited in all examinations, except for 3 patients in the Q group on day 7, who suffered a transient hearing loss greater than 30 dB.
BERA
There was no evidence of drug-induced brain stem lesions by BERA measurements.
Key Points
- There was no detrimental effect of a standard oral regimen of A/L on peripheral hearing or brain stem auditory pathways in patients with uncomplicated falciparum malaria. In contrast, transient hearing loss was common after quinine therapy.
Malaria is a major public health problem in India, accounting for sizeable morbidity, mortality and economic loss. In the past, chloroquine was effective for treating nearly all cases of malaria. In recent studies, chloroquine-resistant P. falciparum malaria has been observed with increasing frequency across the country. The continued treatment of such cases with chloroquine is probably one of the factors responsible for the increased proportion of P. falciparum relative to P. vivax. In addition to chloroquine, P. falciparum is now resistant to several other commonly used antimalarial drugs.
The main mechanism of underlying resistance is a naturally occurring genetic mutation in the parasite that confers a survival advantage. Inadequate treatment (e.g., sub-therapeutic dose, sub-optimal drug) does not kill the parasite and is the main selective pressure for resistance. These resistant parasites are then transmitted to other individuals by mosquitoes, thus increasing the spread of resistance.
To counter this threat of resistance of P. falciparum to monotherapies, and to improve treatment outcomes, combinations of antimalarials with artemisinins are recommended by WHO.
A fixed combination of two antimalarials offers additional important advantages over the free combination of such drugs. Firstly, it facilitates compliance, and secondly, by preventing the patients from taking either drug alone, it helps to prevent the development of resistant strains. A/L is such a fixed-dose combination antimalarial that has been thoroughly evaluated for efficacy and safety in adults and children (including infants).
A/L is an efficacious, well tolerated, WHO-recommended ACT for treating uncomplicated falciparum malaria.
Artemether and Lumefantrine Tablets
LUMET 80
Each uncoated tablet contains:
Artemether ...... 80 mg
Lumefantrine ...... 480 mg
Pharmacodynamics
Artemether is rapidly metabolized into an active metabolite, dihydroartemisinin (DHA). The antimalarial activity of artemether and DHA has been attributed to endoperoxide moiety. The exact mechanism by which lumefantrine exerts its anti-malarial effect is not well defined. Available data suggest that lumefantrine inhibits the formation of beta-hematin by forming a complex with hemin. Both artemether and lumefantrine were shown to inhibit nucleic acid and protein synthesis.
Pharmacokinetics
Absorption
Following administration of artemether/lumefantrine tablets to healthy volunteers and patients with malaria, artemether is absorbed with peak plasma concentrations reached about 2 hours after dosing. Absorption of lumefantrine, a highly lipophilic compound, starts after a lag-up to 2 hours, with peak plasma concentrations about 6 to 8 hours after administration.
Food enhances the absorption of both artemether and lumefantrine. In healthy volunteers, the relative bioavailability of artemether was increased between 2° to 3 fold, and that of lumefantrine 16-fold when artemether/lumefantrine tablets were taken after a high-fat meal, compared under fasted conditions. Patients should be encouraged to take artemether/ lumefantrine tablets with a meal as soon as food can be tolerated.
Distribution
Both artemether and lumefantrine are highly bound to human serum proteins in vitro (95.4% and 99.7%, respectively). The artemether metabolite, DHA, is also bound to human serum proteins (47-76%). Protein binding to human plasma proteins is linear.
Metabolism
In human liver microsomes and recombinant cytochrome (CY) P450 enzymes, the metabolism of artemether was catalyzed predominantly by CYP3A4/5. DHA is an active metabolite of artemether. The metabolism of artemether was also catalyzed to a lesser extent by CYP2B6, CYP2C9 and CYP2C19. In vitro studies with artemether at therapeutic concentrations revealed no significant inhibition of the metabolic activities of CYP1A2, CYP2A6, CYP2C9, CYP2C19, CY12D6, CYP2E1, CYP3A4/5 and CYP4A9/11.
During repeated administration of artemether/lumefantrine tablets, the systemic exposure of artemether decreased significantly, while concentrations of DHA increased, although not to a statistically significant degree. The artemether/DHA area under the curve (AUC) ratio is 1.2 after a single dose and 0.3 after six doses given over 3 days. This suggests that there was induction of CYP3A4/5, which was responsible for the metabolism of artemether.
In human liver microsomes and in recombinant CYP450 enzymes, lumefantrine was metabolized mainly by CYP3A4 to desbutyl-Iumefantrine. The systemic exposure to the metabolite, desbutyl-Iumefantrine, was less than 1% of the exposure to the parent compound. In vitro, lumefantrine significantly inhibits the activity of CYP2D6 at therapeutic plasma concentrations.
Caution is recommended when combining artemether/lumefantrine tablets with substrates, inhibitors or inducers of CYP3A4, especially antiretroviral(ART) drugs and those that prolong the QT interval (e.g., macrolide antibiotics, pimozide, terfenadine, astemizole, cisapride).
Co-administration of artemether and lumefantrine tablets with CYP2D6 substrates may result in increased plasma concentrations of the CYP2D6 substrate and increase the risk of adverse reactions. In addition, many of the drugs metabolized by CYP2D6 can prolong the QT interval and should not be administered with artemether/lumefantrine tablets due to the potential additive effect on the QT interval (e.g., flecainide, imipramine, amitriptyline, clomipramine.
Elimination
Artemether and DHA are cleared from plasma with an elimination half-life of about 2 hours. Lumefantrine is eliminated more slowly, with a terminal half-life of 3-6 days in healthy volunteers and in patients with falciparum malaria. Demographic characteristics such as sex and weight appear to have no clinically relevant effects on the pharmacokinetics of artemether and lumefantrine.
No urinary excretion data are available for humans. In animal studies, artemether metabolites were largely excreted in the urine. However, urinary excretion of artemether, lumefantrine and lumefantrine metabolites was negligible. While animal data are informative, they do not always predict human results.
Hepatic and Renal Impairment
No specific pharmacokinetic studies have been performed in patients with either hepatic or renal impairment.
Geriatric Patients
No specific pharmacokinetic studies have been performed in patients older than 65 years of age.
LUMET 80 tablets are indicated for treatment of acute, uncomplicated malaria infections due to Plasmodium falciparum (P. falciparum) in adults and children above 35 kg. Artemether/ lumefantrine tablets have been shown to be effective in geographical regions where resistance to chloroquine has been reported.
LUMET 80 tablets should not be used to treat patients with severe or complicated P. falciparum malaria.
LUMET 80 tablets should not be used for the prevention of malaria.
Administration Instructions
LUMET 80 tablets should be taken with food. Patients with acute malaria are frequently averse to food. Patients should be encouraged to resume normal eating as soon as food can be tolerated since this improves the absorption of artemether and lumefantrine.
In the event of vomiting within 1 to 2 hours of administration, a repeat dose should be taken. If the repeat dose is vomited, the patient should be given an alternative antimalarial for treatment.
Dosage in Adult Patients (>16 Years of Age)
A 3-day treatment schedule with a total of six doses is recommended for adult patients with a body weight of 35 kg and above:
One tablet as a single initial dose, one tablet again after 8 hours and, then, one tablet twice daily (morning and evening) for the following 2 days (total course of six tablets).
Dosage in Pediatric Patients (≥ 35 Kg)
A 3-day treatment schedule with a total of six doses as described above is recommended for children weighing 35 kg and above.
Dosage in Patients with Hepatic or Renal Impairment
No specific pharmacokinetic studies have been carried out in patients with hepatic or renal impairment. Most patients with acute malaria present with some degree of related hepatic and/ or renal impairment. In clinical studies, the adverse event profile did not differ in patients with mild or moderate hepatic impairment compared to patients with normal hepatic function. No specific dose adjustments are needed for patients with mild or moderate hepatic impairment.
In clinical studies, the adverse event profile did not differ in patients with mild or moderate renal impairment compared to patients with normal renal function. There were few patients with severe renal impairment in clinical studies. There is no significant renal excretion of lumefantrine, artemether and dihydroartemisinin (DHA) in healthy volunteers and while clinical experience in this population is limited, no dose adjustment is recommended.
Caution should be exercised when administering artemether/lumefantrine tablets to patients with severe hepatic or renal impairment.
Artemether/lumefantrine tablets are contraindicated in patients hypersensitive to artemether, lumefantrine or to any of the excipients of the tablet.
Prolongation of the QT Interval
Some antimalarials (e.g., halofantrine, quinine, quinidine), including artemether/lumefantrine tablets, have been associated with prolongation of the QT interval on the electrocardiogram.
Artemether/lumefantrine tablets should be avoided in patients with congenital prolongation of the QT interval (e.g., long QT syndrome), or with any other clinical condition known to prolong the QTc interval such as a history of symptomatic cardiac arrhythmias, with clinically relevant bradycardia or with severe cardiac disease; with a family history of congenital prolongation of the QT interval or sudden death; with known disturbances of electrolyte balance, e.g., hypokalemia or hypomagnesemia; receiving other medications that prolong the QT interval, such as class IA (quinidine, procainamide, disopyramide), or class III (amiodarone, sotalol) anti-arrhythmic agents; antipsychotics (pimozide, ziprasidone); antidepressants; certain antibiotics (macrolide antibiotics, fluoroquinolone antibiotics, imidazole, and triazole antifungal agents); certain non- sedating antihistaminics (terfenadine, astemizole), or cisapride; receiving medications that are metabolized by the, CYP2D6 enzyme, and which also have cardiac effects (e.g., flecainide, imipramine, amitriptyline, clomipramine).
Use of QT-Prolonging Drugs and Other Antimalarials
Halofantrine and artemether/lumefantrine tablets should not be administered within 1 month of each other due to the long elimination half-life of lumefantrine (3-6 days) and potential additive effects on the QT interval.
Antimalarials should not be given concomitantly with artemether/lumefantrine tablets unless there is no other treatment option, due to limited safety data.
Drugs that prolong the QT interval, including antimalarials such as quinine and quinidine, should be used cautiously following artemether/lumefantrine tablets, due to the long elimination half-life of lumefantrine (3-6 days) and the potential for additive effects on the QT interval.
If mefloquine is administered immediately prior to artemether/lumefantrine, there may be a decreased exposure to lumefantrine, possibly due to a mefloquine-induced decrease in bile production. Therefore, patients should be monitored for decreased efficacy and food consumption should be encouraged while taking artemether/lumefantrine tablets (see "Drug Interactions").
Drug Interactions with CYP3A4
When artemether/lumefantrine tablets are co-administered with substrates of CYP3A4 it may result in decreased concentrations of the substrate and potential loss of substrate efficacy. When artemether/lumefantrine tablets are coadministered with an inhibitor of CYP3A4, including grapefruit juice it may result in increased concentrations of artemether and/or lumefantrine and potentiate QT prolongation. When artemether/lumefantrine tablets are co-administered with inducers of CYP3A4 it may result in decreased concentrations of artemether and/or lumefantrine and loss of anti-malarial efficacy (see "Drug Interactions").
Drugs that have a mixed effect on CYP3A4, especially Anti-Retroviral drugs, and those that have an effect on the QT interval should be used with caution in patients taking artemether/ lumefantrine tablets (see "Drug Interactions").
Artemether/lumefantrine tablets may reduce the effectiveness of hormonal contraceptives. Therefore, patients using oral, transdermal patch, or other systemic hormonal contraceptives should be advised to use an additional nonhormonal method of birth control (see "Drug Interactions").
Drug Interactions with CYP2D6
Administration of artemether/lumefantrine tablets with drugs that are metabolized by CYP2D6 may significantly increase plasma concentrations of the co-administered drug and increase the risk of adverse effects. Many of the drugs metabolized by CYP2D6 can prolong the QT interval and should not be administered with artemether/lumefantrine tablets due to the potential additive effect on the QT interval (e.g., flecainide, imipramine, amitriptyline, clomipramine) (see "Drug Interactions").
Recrudescence
Food enhances the absorption of artemether and lumefantrine following administration of artemether/lumefantrine tablets. Patients who remain averse to food during treatment should be closely monitored as the risk of recrudescence may be greater.
In the event of recrudescent P. falciparum infection after treatment with artemether/lumefantrine tablets, patients should be treated with a different antimalarial drug.
Plasmodium Vivax (P. Vivax) Infection
Artemether/lumefantrine tablets have been shown in limited data (43 patients) to be effective in treating the erythrocytic stage of P. vivax infection. However, relapsing malaria caused by P. vivax requires additional treatment with other antimalarial agents to achieve a radical cure, i.e., eradicate any hypnozoites forms that may remain dormant in the liver.
Ketoconazole
In a study of 15 healthy subjects, concurrent oral administration of ketoconazole, a potent CYP3A4 inhibitor, with a single dose of artemether/lumefantrine tablets resulted in a moderate increase in exposure to artemether, DHA (the metabolite of artemether), and lumefantrine. No dose adjustment of artemether/lumefantrine tablets is necessary when administered with ketoconazole or other potent CYP3A4 inhibitors. However, due to the potential for increased concentrations of lumefantrine, which could lead to QT prolongation, artemether/lumefantrine tablets should be used cautiously with drugs that inhibit CYP3A4 (e.g. grapefruit juice).
Prior Use of Mefloquine
Administration of 3 doses of mefloquine followed 12 hours later by a six-dose regimen of artemether/lumefantrine tablets in 14 healthy volunteers demonstrated no effect of mefloquine on plasma concentrations of artemether or the artemether/DHA ratio. However, exposure to lumefantrine was reduced, possibly due to lower absorption secondary to a mefloquine-induced decrease in bile production. Patients should be monitored for decreased efficacy and food consumption should be encouraged with the administration of artemether/lumefantrine tablets.
CYP3A4 Metabolism: Hormonal Contraceptives and Anti-Retroviral Drugs Aremether induces CYP3A4 and both artemether and lumefantrine are metabolized primarily by CYP3A4.
Artemether/lumefantrine tablets may reduce the effectiveness of hormonal contraceptives. Therefore, patients using an oral transdermal patch, or other systemic hormonal contraceptives should be advised to use an additional non-hormonal method of birth control.
Anti-Retroviral drugs such as protease inhibitors and non-nucleoside reverse transcriptase inhibitors are known to have variable patterns of inhibition, induction or competition for CYP3A4. No formal drug-drug interaction studies between artemether/lumefantrine tablets and Anti-Retroviral drugs have been performed. However, artemether/lumefantrine tablets should be used cautiously in patients on Anti-Retroviral drugs as the result may be an increase in lumefantrine concentrations, causing QT prolongation, or a decrease in concentrations of the antiretroviral drugsresulting in loss of efficacy or a decrease in artemether and/or lumefantrine concentrations, resulting in loss of the antimalarial efficacy of artemether/lumefantrine tablets.
CYP2D6 Substrates
Lumefantrine inhibits CYP2D6 in vitro. Administration of artemether/lumefantrine tablets with drugs that are metabolized by CYP2D6 may significantly increase plasma concentrations of the co-administered drug and increase the risk of adverse effects. Many of the drugs metabolized by CYP2D6 can prolong the QT interval and should not be administered with artemether/ lumefantrine tablets due to the potential additive effect on the QT interval (e.g., flecainide, imipramine, amitriptyline, clomipramine).
Sequential Use of Quinine
A single dose of intravenous quinine (10 mg/kg body weight) concurrent with the final dose of a 6-dose regimen of artemether/lumefantrine tablets demonstrated no effect of intravenous quinine on the systemic exposure of DHA or lumefantrine. Quinine exposure was also not altered. Exposure to artemether was decreased. This decrease in artemether exposure is no thought to be clinically significant. However, quinine and other drugs that prolong the QT interval should be used cautiously following treatment with artemether/lumefantrine tablets due to the long elimination half-life of lumefantrine and the potential for additive QT effects.
Pregnancy Category C
Safety data from an observational pregnancy study of approximately 500 pregnant women who were exposed to artemether/lumefantrine tablets (including a third of patients who were exposed in the first trimester), and published data of over 1000 pregnant patients who were exposed to artemisinin derivatives, did not show an increase in adverse pregnancy outcomes or teratogenic effects over the background rate.
The efficacy of artemether/lumefantrine tablets in the treatment of acute, uncomplicated malaria in pregnant women has not been established.
Artemether/lumefantrine tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
It is not known whether artemether or lumefantrine is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when artemether/lumefantrine tablets are administered to a nursing mother. Animal data suggest that both artemether/ lumefantrine are excreted into breast milk. The benefits of breastfeeding to the mother and the infant should be weighed against the potential risks from exposure of the infant to artemether/ lumefantrine through breast milk.
Lumet 80 can be used in children weighing 35 kg and above.
Clinical studies of artemether/lumefantrine tablets did not include sufficient numbers of subjects aged 65 years and over to determine if they respond differently from younger subjects. In general, the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy in elderly patients should be considered when prescribing artemether/lumefantrine tablets.
Artemether/lumefantrine tablets have not been studied for efficacy and safety in patients with severe hepatic and/or renal impairment see "Dosage and Administration").
Hypersensitivity reactions have been reported during postmarketing surveillance.
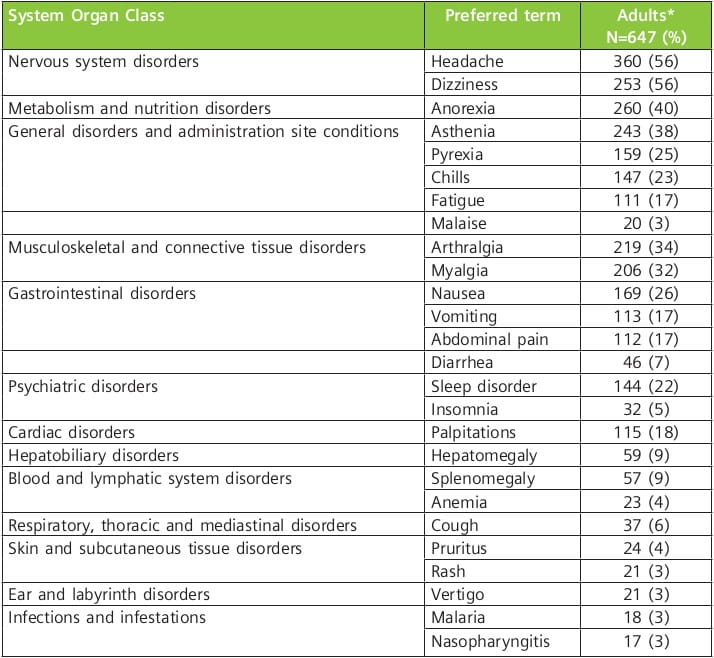
The adverse reactions occurring in 3% or more of adult patients treated in clinical trials with the 6-dose regimen of artemether/lumefantrine tablets are as follows:
The following adverse reactions have been identified during post-approval use of artemether/ lumefantrine tablets. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
Hypersensitivity, including urticaria and angioedema. Serious skin reactions (bullous eruption) have been rarely reported.
There is no information on overdoses of artemether/lumefantrine tablets higher than the doses recommended for treatment.
In cases of suspected overdosage, symptomatic and supportive therapy, which would include ECG and blood electrolyte monitoring, should be given as appropriate.
LUMET 80 Tablets - Blister pack of 6 tablets
Last updated: August 2010
1. World Malaria Report 2009. World Health Organisation. http://www.who.int/malaria/publications/atoz/9789241563901/en/index.html. Last accessed on 11.08.2010. 2. http://nvbdcp.gov.in/Doc/Malaria-situation-may2010.pdf. Last accessed on 11.08.2010.
3. Guidelines for the Treatment of Malaria, World Health Organisation, 2010. http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf. Last accessed on 11.08.2010.
4. Pauline Byakika-Kibwika, Mohammed Lamorde et al. Update on the efficacy, effectiveness and safety of artemether/lumefantrine combination therapy for treatment of uncomplicated malaria. Therapeutics and Clinical Risk Management 2010; 6:11-20.
5. Drug Resistance. www.malariasite.com/malaria/DrugResistance.htm. Last accessed on 27.07.2010.
6. Francois Nosten, Philippe Brasseur et al. Combination Therapy for Malaria. Drugs 2002; 62 (9):1315-1329.
7. Guidelines for The Treatment Of Malaria, World Health Organization, 2006. http://whqlibdoc.who.int/publications/2006/9241546948_text_eng.pdf. Last accessed on 11.08.2010.
8. M. Geyer. Antimalarial Drug Combination Therapy - Report of a WHO Consultation 2001. http://whqlibdoc.who.int/hq/2001/WHO_CDS_RBM_2001.35.pdf. Last accessed on 11.08.2010.
9. Nicholas J. White, Michele van Vugt et al. Clinical Pharmacokinetics and Pharmacodynamics of Artemether/Lumefantrine. Clinical Pharmacokinet 1999 Aug; 37(2): 105-125.
10. Martin Meremikwu, Ambrose Alaribe et al. Artemether/Lumefantrine versus Artesunate + Amodiaquine for Treating Uncomplicated Childhood Malaria in Nigeria: Randomized Controlled Trial. Malar J. 2006; 5: 43.
11. Neena Valecha, Prakriti Srivastava et al. Therapeutic efficacy of artemether/lumefantrine in uncomplicated falciparum malaria in India. Malaria Journal 2009; 8:107.
12. Robert Hutagalung, Lucy Paiphun et a. Randomized Trial of Artemether/Lumefantrine versus Mefloquine/Artesunate for the Treatment of Uncomplicated Multi-drug Resistant Plasmodium Falciparum on the Western Border of Thailand. Malaria Journal 2005; 4:46-54.
13. Babacar Faye, Jean-Louis Ndiaye et al. Efficacy and tolerability of four antimalarial combinations in the treatment of uncomplicated Plasmodium falciparum malaria in Senegal. Malaria Journal 2007; 6:80.
14. Alecrim MG, Lacerda MV et al. Successful Treatment of Plasmodium Falciparum Malaria with a Six-Dose Regimen of Artemether/Lumefantrine versus Quinine-Doxycycline in the Western Amazon Region of Brazil. Am J Trop Med Hyg. 2006 Jan; 74(1): 20-5.
15. Mayfong Mayxay, Maniphone Khanthavong et al. Randomized Comparison of Chloroquine + Sulphadoxine-Pyrimethamine versus Artesunate + Mefloquine versus Artemether/Lumefantrine in the Treatment of Uncomplicated Falciparum Malaria in the Laos People's Democratic Republic. Clinical Infectious Diseases 2004; 39:1139-47.
16. Bindschedler M, Lefevre G et al. Comparison of the Cardiac Effects of the Antimalarials, Co- Artemether and Halofantrine in Healthy Participants. Am J Trop Med Hyg 2002 Mar; 66(3): 293-8.
17. Robert Gurkov, Teferi Eshetu et al. Ototoxicity of artemether/lumefantrine in the treatment of falciparum malaria: a randomised trial. Malaria Journal 2008; 7:179.