Opportunistic Infections
Fact Sheets
Human immunodeficiency virus (HIV) causes a chronic infection that leads to profound immunosuppression. A
hallmark of this process is the depletion of CD4 lymphocytes, and this predisposes the patient to develop a
variety of opportunistic infections and certain neoplasms.
The course of the infection may vary, with some individuals developing immunodeficiency within 2 to 3 years and
others remaining asymptomatic for 10 to 15 years. A typical course spanning over about 10 years is depicted in
the following chart:
Thus, the CD4 T-lymphocyte count is the best-validated predictor of the likelihood of developing an opportunistic
infection. Susceptibility to opportunistic infections increases as HIV-induced immunodeficiency becomes more
severe.
Management of HIV infection involves treating the opportunistic infections, as well as inhibiting viral
replication using antiretrovirals. It is important to note that drug interactions may occur between the various
drugs in the antiretroviral regimen, as well as between antiretrovirals and drugs used to treat the
opportunistic infections. A pertinent example is the interaction between rifampicin and antiretrovirals
(discussed under "Tuberculosis"). An excellent online tool to ascertain possible drug interactions is
available at www. hiv-druginteractions. org.
Mycobacterium tuberculosis
May be pulmonary or extra-pulmonary, such as lymphadenitis, meningitis etc. Cough, weight loss, night sweats,
fever, swollen lymph nodes, or organ-specific symptoms. However, extrapulmonary disease is more common in
HIV-1-infected persons than in non-HIV-1-infected persons.
- Chest radiography.
- Ultrasonography or CT scan for extra-pulmonary TB.
- Sputum samples for AFB smear and culture obtained from patients with pulmonary symptoms, cervical adenopathy
or chest radiographic abnormalities.
- Among patients with signs of extrapulmonary TB, needle aspiration of skin lesions, nodes, pleural or
pericardial fluid might allow for rapid diagnosis, culture, and susceptibility testing.
- Tissue biopsy is helpful among patients with negative fine-needle aspirates.
- Among patients with signs of disseminated disease, mycobacterial blood culture is diagnostic.
Tuberculous Pleural Effusion
Obliteration of right costophrenic angle in a patient with early HIV disease
A number of potential problems can be encountered if antiretroviral therapy (ART) and anti-TB therapy (AKT) are
prescribed concurrently. These include overlapping toxicities, increased pill burden and risk of sub-optimal
adherence, drug-drug interactions and inflammatory reactions.
For patients with active TB in whom HIV infection is diagnosed and ART is required, the first priority is to
initiate standard anti-TB treatment. The optimal time to initiate ART is not known. Case-fatality rates in
patients with TB during the first two months of TB treatment are high, particularly in settings with a high
prevalence of HIV, suggesting that ART should begin early. On the other hand, considerations of pill burden,
drug-drug interactions, toxicity, and immune reconstitution inflammatory syndrome (IRIS) support the later
initiation of ART
Thus, a balance needs to be established between the risk of disease progession and death it ART is deferred,
against an increased risk of inflammatory reactions and other adverse events if ART is started rapidly after TB
diagnosis.
The WHO (2006) recommendations are as follows:
In patients with CD4 counts <200 cells/mm3: ART should be started as soon as the patient
has tolerated and stabilized on TB treatment. It usually takes between 2 and 8 weeks after the start of TB
treatment.
In patients with CD4 counts >200 ceils/mm3: Initiation of ART may be delayed until after
the initial intensive phase of TB treatment has been completed.
In patients with CD4 counts >350 cells/mm3: ART can be delayed until after the
completion of short-course TB therapy, following a reassessment of the patient's eligibility for ART and
evaluation of the response to TB therapy and of CD4 counts, if available.
A fungal infection.
The occurrence of pharyngeal or esophageal candidiasis is recognized as an indicator of immune suppression, and
these are most often observed in patients with CD4 counts < 200 cells/μL. In contrast, vulvovaginal
candidiasis is common among healthy, adult women and is unrelated to HIV-1 status.
Candida albicans is the predominant causative agent of all forms of mucocutaneous candidiasis. Less
frequently, C. glabrata, C. parapsilosis, C. tropicalis, C. kruseii and several other species may cause
candidiasis.
Oropharyngeal Candidiasis
Oropharyngeal candidiasis is characterized by painless, creamy white, plaque-like lesions of the buccal or
oropharyngeal mucosa or tongue surface. Lesions can be easily scraped off with a tongue depressor or other
instrument. Less commonly, erythematous patches without white plaques can be seen on the anterior or posterior
upper palate or diffusely on the tongue. Angular chelosis is also noted on occasion.
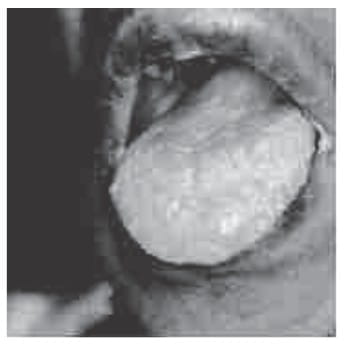
Oral Candidiasis (thrush) in intermediate HIV disease
Numerous superficial white pustules that 'peel off' as a white membrane leaving behind erosions.
Esophageal Candidiasis
Esophageal candidiasis is occasionally asymptomatic but often presents with fever, retrosternal burning pain or
discomfort, and odynophagia.
Vulvovaginal Candidiasis
Vulvovaginitis is characterized by a creamy to yellow-white adherent vaginal discharge associated with mucosal
burning and itching.
Oropharyngeal Candidiasis
Visual examination, ability to scrape off the superficial whitish plaques.
Esophageal Candidiasis
Usually diagnosed presumptively if dysphagia and odynophagia are present with thrush. Alternatively, upper Gl
tract endoscopy followed by histopathologic demonstration and culture confirmation.
Vulvovaginal Candidiasis
Vulvovaginal candidiasis is based on clinical presentation coupled with the demonstration of characteristic
yeast forms in vaginal secretions examined microscopically after KOH preparation.
Oropharyngeal Candidiasis
Although initial episodes of oropharyngeal candidiasis can be adequately treated with topical therapy, including
clotrimazole troches or nystatin suspension, oral fluconazole (200 mg on the first day, followed by 100 mg once
daily for 2 weeks) is as effective, superior to topical therapy, more convenient and generally better tolerated.
Itraconazole oral solution for 7-14 days is as effective as oral fluconazole but less well tolerated.
Ketoconazole and itraconazole capsules are less effective than fluconazole because of their more variable
absorption and should be considered second line alternatives.
Esophageal Candidiasis
Systemic therapy is required for effective treatment of esophageal candidiasis. A 14-21 day course of either
fluconazole (200 mg on the first day, followed by 100 mg once daily for 3 weeks) or itraconazole solution is
highly effective. As with oropharyngeal candidiasis, ketoconazole and itraconazole capsules are less effective
than fluconazole because of variable absorption.
Vulvovaginal Candidiasis
Responds readily to short-course oral or topical treatment with any of several therapies including single-dose
regimens:
- Topical azoles (clotrimazole, butaconazole, miconazole, ticonazole, or terconazole)
- Topical nystatin
- Itraconazole oral solution
- Oral fluconazole (150 mg as a single dose)
Fluconazole Refractory Oropharyngeal Candidiasis
Responds at least transiently to itraconazole solution or amphotericin B oral suspension or intravenous
amphotericin B.
Fluconazole Refractory Esophageal Candidiasis
Responds to caspofungin or intravenous amphotericin B.
Majority of HIV specialists do not recommend secondary prophylaxis (chronic maintenance therapy).
Varicella Zoster Virus (VZV).
VZV causes 2 clinically distinct diseases. Varicella (or chickenpox) is a common and extremely contagious acute
illness that occurs in epidemics among school-aged children and is characterized by a generalized vesicular
rash. VZV establishes latency following primary infection. Reactivation of latent VZV results in herpes zoster
(or shingles), a localized cutaneous eruption that is most common among the elderly.
Complications of both varicella and herpes zoster are more frequent in immunocompromised patients. The incidence
of herpes zoster is greater in HIV-infected patients than that in the general population and can occur at any
CD4 count.
Herpes zoster (Shingles)
Herpes zoster might follow a prodrome of pain that resembles a burn or muscle injury in the affected dermatome:
skin lesions, which are similar to chickenpox in appearance and evolution, develop in the same dermatome. In a
patient with a damaged immune system as in HIV/AIDS, it may involve multiple dermatomes.
Ocular zoster
Acute retinal necrosis occurs as a peripheral necrotizing retinitis with yellowish thumbprint lesions, retinal
vascular sheathing, and vitritis with a high rate of visual loss, often caused by retinal detachment.
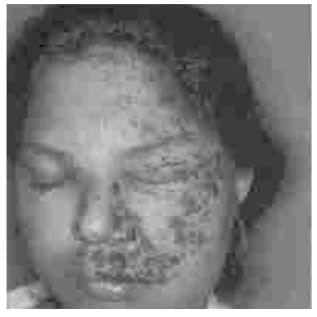
Herpes Zoster Ophthalmicus
Haemorrhagic vesicles and erosions on a background of erythema and edema in early stage HIV disease. Note
extension to maxillary branch.
Varicella (Chickenpox)
Chickenpox, the principal clinical manifestation of primary VZV in childhood or adulthood is uncommon in adults
and adolescents with HIV-1 infection. When chickenpox occurs, it begins with a respiratory prodrome, followed by
the appearance of pruritic vesiculopapular lesions that are more numerous on the face and trunk than on the
extremities. Lesions evolve over a 5-day period through macular, papular, vesicular pustular and crust stages.
In profoundly immunocompromised hosts, vesicles can persist for weeks and coalesce to form large lesions that
resemble a burn.
Other
VZV has been associated with transverse myelitis, encephalitis, and vasculitic stroke among HIV-uninfected
persons.
Herpes zoster
The characteristic vesicular rash usually makes it an obvious clinical diagnosis. If the presentation is
atypical, the diagnosis can be best confirmed by testing a smear of a swab of the base of a skin lesion for the
presence of VZV antigen by a direct fluorescent antibody method.
Ocular zoster
VZV retinitis is a clinical diagnosis made by the typical clinical appearance of the retina and confirmed by a
history of concurrent or recent cutaneous zoster.
Varicella
Distinctive appearance - a clinical d iagnosis is usually accurate.
Herpes zoster
The recommended treatment for localized dermatomal herpes zoster is acyclovir, famciclovir or valacyclovir for
7-10 days. If cutaneous lesions are extensive or if clinical evidence of visceral involvement is observed,
intravenous acyclovir should be initiated and continued until cutaneous lesions and visceral disease are clearly
resolving.
Ocular zoster
Progressive outer retinal necrosis is rapidly progressive. Recommended treatment is high-dose intravenous
acyclovir in combination with foscarnet. Concomitant laser retinal photocoagulation might be needed to prevent
retinal detachments.
Varicella
Intravenous acyclovir for 7-10 days is the recommended initial treatment for adults and adolescents with
chickenpox. Switching to oral therapy after the patient has defervesced if no evidence of visceral involvement
exists might be permissible.
Successful treatment with intravenous acyclovir has been reported.
Among patients with suspected or proven acyclovir-resistant VZV infections, treatment with
intravenous foscarnet is the recommended alternative therapy.
No drug has been proven to prevent the recurrence of zoster (shingles) among HIV-1 infected persons.
Herpes simplex virus type 1 (HSV-1) and Herpes simplex virus type 2(HSV-2)
HSV orolabialis
HSV orolabialis is the most common manifestation of HSV-1 infection presenting with a sensory prodrome in the
affected area, rapidly followed by the evolution of lesions from papule to vesicle, ulcer, and crust stages on
the lips. Ulcerative lesions are usually the only stage observed on mucosal surfaces. The course of illness in
untreated subjects is 7-10 days. Lesions recur 1 -12 times per year and are often triggered by sunlight or
stress.
HSV genitalis
HSV genitalis is the more common manifestation of HSV-2 infection. Perineal lesions on keratinylated skin are
similar in appearance and evolution to external orofacial lesions. Local symptoms include a sensory prodrome
consisting of pain and pruritis. Ulcerative lesions are usually the only stage observed on vaginal or urethral
mucosal surfaces.
Mucosal disease is generally accompanied by dysuria, vaginal or uretheral discharge; inguinal lymphadenopathy,
particularly in primary infection, is common with perineal disease.
Others
HSV keratitis, neonatal HSV, HSV encephalitis, and herpetic whitlow are similar in presentation and treatment to
those diseases observed in HIV-seronegative persons but might be more severe.
Diagnostic Procedures
HSV infections are usually diagnosed on the basis of characteristic skin, mucous membrane, or ophthalmic
lesions. Smear, viral culture, or HSV antigen detection can confirm the diagnosis.
Herpes Simplex
Typical grouped vesicular lesions of herpes simplex are seen at an unusual site in early stage HIV disease.
Scars of previous attack of herpes simplex are seen in the same region. Differential diagnosis includes
recurrent herpes zoster affecting the same dermatome.
HSV orolabialis
Orolabial lesions can be treated with oral famciclovir, valacyclovir, or acyclovir for 7 days.
Moderate-to-severe mucocutaneous HSV lesions are best treated initially with intravenous acyclovir. Patients may
be switched to oral therapy after the lesions have completely healed.
HSV genitalis
Initial or recurrent genital HSV should be treated with oral famciclovir, valacyclovir or acyclovir for 7-14
days.
HSV keratitis
Trifluridine is the treatment of choice for herpes keratitis, one drop onto the cornea every 2 hours, not to
exceed 9 drops/day; it is not recommended for longer than 21 days.
HSV encephalitis
Intravenous acyclovir, 10 mg/kg body weight every 8 hours for 14-21 days, is required for HSV encephalitis.
The treatment of choice for acyclovir-resistant HSV is IV foscarnet. Topical trifluridine or cidofovir also has
been used successfully for lesions on external surfaces, although prolonged application for 21 - 28 days or
longer might be required.
Persons who have frequent or severe recurrences can be administered daily suppressive therapy with oral
acyclovir, oral famciclovir, or oral valacyclovir. Intravenous foscarnet or cidofovir can be used to treat
infection caused by acyclovir-resistant isolates of HSV.
- Bacteria: Salmonella, Shigella, Campylobacter
- Parasitic infections: Cryptosporidium, Isospora, Giardia, Microsporidia, Entamoeba histolytica
- Mycobacterial infections: Mycobacterium avium complex (MAC), Mycobacterium tuberculosis
- Viral infections: Cytomegalovirus
- Drug-associated diarrhoea: Certain antiretrovirals such as protease inhibitors (e.g. nelfinavir)
commonly cause diarrhoea
- Idiopathic diarrhoea, often labelled 'HIV enteropathy'
Diarrhoea results from either small intestinal or colonic pathologic conditions. A careful history and physical
examination will direct the evaluation and treatment strategy of AIDS-associated diarrhoea.
Small-intestinal disease produces large volume diarrhoea that is frequently associated with dehydration and serum
electrolyte abnormalities. Abdominal pain, gaseous distension, nausea and vomiting also may be present. Tenesmus
and fecal leukocytes are absent.
Colonic diarrhoea is less voluminous, and dehydration is uncommon. Tenesmus and left lower quadrant pain are
common.
Bacterial infections
Salmonella, Shigella and Campylobacter cause more severe diarrhoea with longer duration of
illness in the immunocompromised host. The diagnosis is established through cultures of stool and blood.
Endoscopy may be useful.
Parasitic infections
Cryptosporidium, Isospora, Giardia and Microsporidia all infect the small intestine.
Entamoeba histolytica is uncommon but when present involves the caecum, ascending colon and terminal
ileum. Strongyloides stercoralis may also be encountered. The diagnosis can be established through
microscopic identification of oocytes in stool or tissue.
Viral Infections
Cytomegalovirus (CMV) is the most important viral cause of AIDS-associated diarrhoea. CMV may affect any part of
the Gl tract; colitis and oesophagitis are quite common.
Mycobacterial infections
Mycobacterium avium is found in macrophages in the lamina propria of the small intestine or colon.
Mycobacterium tuberculosis is most commonly found in the caecum and terminal ileum.

Pneumocystis jiroveci is a ubiquitous organism classified as a fungus but which shares biologic
characteristics with protozoa. The taxonomy of the organism has been changed: Pneumocystis carinii now
refers only to the pneumocystis that infects rodents, and Pneumocystis jiroveci refers to the distinct
species that infects humans. The abbreviation PCP is still used to designate Pneumocystis pneumonia.
Subacute onset of progressive exertional dyspnea, fever, nonproductive cough, and chest discomfort that worsens
over a period of days to weeks. Oral thrush is a common co-infection.
Pulmonary examination is usually normal at rest. With exertion, tachypnea, tachycardia, and diffuse dry rales
might be observed.
The chest radiograph typically demonstrates diffuse, bilateral, symmetrical interstitial infiltrates emanating
from the hila in a butterfly pattern.
Histopathologic demonstration of organisms in tissue, bronchoalveolar lavage fluid, or induced sputum.
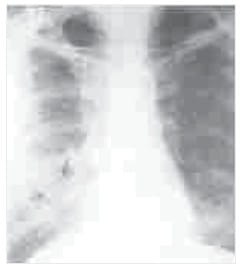
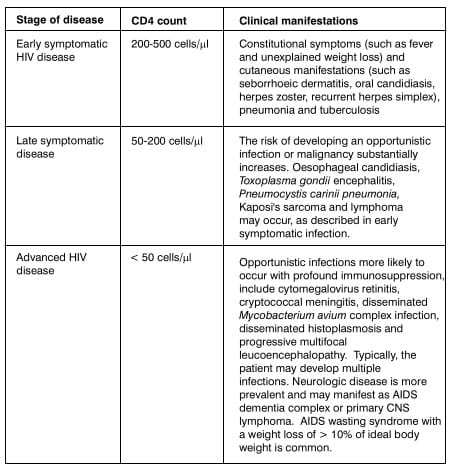
Pneumocystis jiroveci Pneumonia (PCP) Diffuse small acinar shadows (arrows), with ground glass
appearance in both lower lobes suggests PCP
Trimethoprim-sulfamethoxazole (TMP-SMX) is the treatment of choice.
Alternative therapeutic regimens include:
- Dapsone and TMP for mild-to-moderate disease
- Primaquine plus clindamycin
- Intravenous pentamidine
- Atovaquone suspension
- Trimetrexate with leucovorin
For moderate to severe disease, the common practice is to use parenteral pentamidine, primaquine combined with
clindamycin, or trimetrexate (with or without oral dapsone) plus leucovorin. For mild disease, atovaquone is a
reasonable alternative.
Patients who have a history of PCP should be administered secondary prophylaxis (chronic maintenance therapy) for
life with TMP-SMX unless immune reconstitution occurs as a result of ART.
Alternatives are dapsone, dapsone combined with pyrimethamine, atovaquone or aerosolized pentamidine.
Virtually all HIV-1-associated cryptococcal infections are caused by Cryptococcus neoformans var
neoformans.
Cryptococcosis among patients with AIDS most commonly occurs as a subacute meningitis or meningoencephalitis with
fever, malaise and headache. Certain patients might present with encephalopathic symptoms (e.g., lethargy,
altered mentation, personality changes and memory loss).
Disseminated disease is a common manifestation, with or without concurrent meningitis. Approximately half of the
patients with disseminated disease have evidence of pulmonary rather than meningeal involvement. Symptoms and
signs of pulmonary infection include cough or dyspnea and abnormal chest radiographs. Skin lesions might be
observed.
Cryptococcal antigen is almost invariably detected in the CSF. If disseminated or other organ disease is
suspected in the absence of meningitis, a fungal blood culture is also diagnostically helpful. Detection of
cryptococcal antigen in serum might be useful in initial diagnosis.
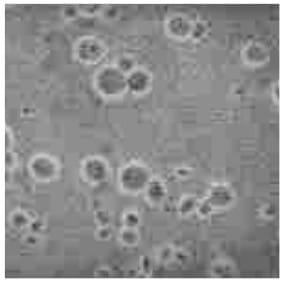
Cryptococcus Smear
India ink preparation brings out the prominent translucent capsule of the organism.
Untreated cryptococcal meningitis is fatal. The recommended initial treatment for acute disease is amphotericin
B, usually combined with flucytosine, for a 2-week duration followed by fluconazole alone for an additional 8
weeks.
Itraconazole is an acceptable though less effective alternative. The principal initial intervention for reducing
symptomatic elevated intracranial pressure is repeated daily lumbar punctures. CSF shunting should be considered
for patients in whom daily lumbar punctures are no longer being tolerated or whose signs and symptoms of
cerebral edema are not being relieved.
The optimal therapy for those with treatment failure is not known. Those who have failed on fluconazole should be
treated with amphotericin B with or without flucytosine as indicated previously, and therapy should be continued
until a clinical response occurs. Higher doses of fluconazole in combination with flucytosine also might be
useful.
Patients who have completed initial therapy for cryptococcosis should be administered lifelong suppressive
treatment (i.e., secondary prophylaxis or chronic maintenance therapy), unless immune reconstitution occurs as a
consequence of ART. Fluconazole is superior to itraconazole for preventing relapse of cryptococcal disease and
is the preferred drug.
The most common clinical presentation of T. gondii infection among patients with AIDS is focal encephalitis with
headache, confusion, or motor weakness and fever. Physical examination might demonstrate focal neurological
abnormalities, and in the absence of treatment, disease progression results in seizures, stupor and coma.
Retinochoroiditis, pneumonia, and evidence of other multifocal organ system involvement can be seen after
dissemination of infection but are rare manifestations in this patient population.
CT scan or MRI of the brain will typically show multiple contrast-enhancing lesions, often with associated edema.
Positron emission tomography (PET) or single-photon emission computed tomography (SPECT) scanning might be
useful for distinguishing between Toxoplasmic encephalitis (TE) and primary central nervous system (CNS)
lymphoma, but no imaging technique is completely specific.
HIV-1 infected patients with toxoplasmic encephalitis are almost uniformly seropositive for anti-toxoplasma IgG
antibodies. Brain biopsy is reserved for patients failing to respond to specific therapy.
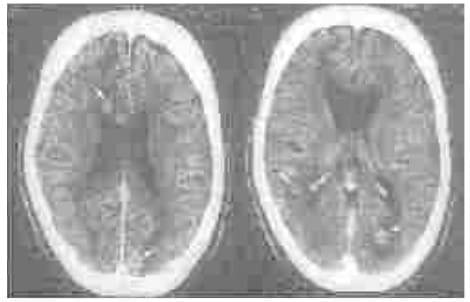
Toxoplasmosis
CT scan-post contrast. Multiple granulomas (arrows) surrounded by minimal vasogenic edema. However, presence of
multiple lesions with a target sign and positive toxoplasma titre suggested toxoplasmosis. Prompt response to a
trial of pyrimethamine and sulfadoxine favoured toxoplasmosis.
Treatment
The initial therapy of choice consists of combination of pyrimethamine plus sulfadiazine plus leucovorin. The
preferred alternative regimen for patients unable to tolerate or who fail to respond to first-line therapy is
pyrimethamine plus clindamycin plus leucovorin.
Management of Treatment Failure
A brain biopsy, if not previously performed, should be strongly considered for patients who fail to respond to
initial therapy.
Prevention of Recurrence
Patients who have successfully completed a 6-week course of initial therapy should be administered lifelong
secondary prophylaxis unless immune reconstitution occurs because of ART
Mycobacterium avium and Mycobacterium intracellular
MAC disease among patients with AIDS, in the absence of ART, is generally a disseminated multiorgan infection.
Early symptoms might be minimal and might precede detectable intermittent or continuous mycobacteremia by
several weeks. Symptoms include fever, night sweats, weight loss, fatigue, diarrhoea and abdominal pain.
Other localized manifestations of MAC disease have been reported most commonly among persons who are receiving
and who have responded to ART. Localized syndromes include cervical or mesenteric lymphadenitis, pneumonitis,
pericarditis, osteomyelitis, skin or soft tissue abscesses, genital ulcers or CNS infection.
Laboratory abnormalities particularly associated with disseminated MAC disease include anaemia (often out of
proportion to that expected for stage of HIV-1 disease) and elevated liver alkaline phosphatase. Hepatomegaly,
splenomegaly or lymphadenopathy (paratracheal, retroperitoneal, para-aortic, or less commonly peripheral) might
be identified on physical examination or by radiographic or other imaging studies.
A confirmed diagnosis of disseminated MAC disease is based on compatible clinical signs and symptoms coupled with
the isolation of MAC from cultures of blood, bone marrow, or other normally sterile tissue or body fluids.
Other ancillary studies provide supportive diagnostic information, including AFB smear and culture of stool or
biopsy material obtained from tissues or organs, radiographic imaging of the abdomen or mediastinum for
detection of lymphadenopathy, or other studies aimed at isolation of organisms from focal infection sites.
Initial treatment of MAC disease should consist of two antimycobacterial drugs to prevent or delay the emergence
of resistance. Clarithromycin is the preferred first agent. It appears to be associated with more rapid
clearance of MAC from the blood.
However, azithromycin can be substituted for clarithromycin when drug interactions or clarithromycin intolerance
preclude the use of clarithromycin.
Ethambutol is the recommended second drug. Some clinicians would add rifabutin as a third drug. The addition of
rifabutin should be considered in persons with advanced immunosuppression (CD4 T lymphocyte
count<50cells/μL), high mycobacterial loads (> 2 log10 colony units/mL of blood), or in the
absence of effective ART, settings in which mortality is increased and emergence of drug resistance is most
likely. If rifabutin cannot be used because of drug interactions or intolerance, a third or fourth drug may be
selected from among eitherthefluoroquinolones (ciprofloxacin orlevofloxacin) or parenteral amikacin.
Patients who have had disseminated MAC disease diagnosed and who have not previously been treated with or are not
receiving potent ART should generally have ART initiated simultaneously or within 1-2 weeks of initiation of
antimycobacterial therapy for MAC disease. If ART has already been instituted, it should be continued and
optimized for patients.
Persons who have symptoms of moderate-to-severe intensity because of an immune recovery inflammatory syndrome in
the setting of ART should receive treatment initially with nonsteroidal, anti-inflammatory agents. If symptoms
fail to improve, short-term (4-8 weeks) systemic corticosteroid therapy has been successful.
Because the number of drugs with demonstrated clinical activity against MAC is limited, results of susceptibility
testing should be used to construct a new multidrug regimen consisting of at least two new drugs not previously
used and to which the isolate is susceptible from among the following: ethambutol, rifabutin, ciprofloxacin or
levofloxacin, or amikacin. Whether continuing clarithromycin or azithromycin in the face of resistance provides
additional benefit is unknown. Among patients who have failed initial treatment for MAC disease or who have
antimycobacterial drug resistant MAC disease, optimizing ART is an important adjunct to secondary or salvage
therapy for MAC disease.
Adult and adolescent patients with disseminated MAC disease should receive lifelong secondary prophylaxis, unless
immune reconstitution occurs as a result of ART.
Cytomegalovirus is a member of the herpes family of viruses. Majority of infections derive from reactivation of
latent infection.
Retinitis
Retinitis is the most common clinical manifestation of CMV end-organ disease. CMV retinitis usually occurs as
unilateral disease, but in the absence of therapy, viremic dissemination results in bilateral disease in the
majority of patients.
Peripheral retinitis might be asymptomatic or present with floaters, scotomata, or peripheral visual field
defects. Central retinal lesions or lesions impinging on the macula are associated with decreased visual acuity
or central field defects. The characteristic ophthalmologic appearance of CMV lesions includes perivascular
fluffy yellow-white retinal infiltrates, typically described as a focal necrotizing retinitis, with or without
intraretinal haemorrhage, and with little inflammation of the vitreous humour unless immune recovery with potent
ART intervenes. Blood vessels near the lesions might appear to be sheathed. Occasionally, the lesions might have
a more granular appearance.
Colitis
Colitis is the second most common manifestation. It causes fever, weight loss, anorexia, abdominal pain,
debilitating diarrhoea, and malaise.
Esophagitis
Esophagitis caused by CMV occurs in persons with AIDS who develop CMV end-organ disease, and causes fever,
odynophagia, nausea, and occasionally mid-epigastric or retrosternal discomfort.
Pneumonitis
CMV pneumonitis is uncommon, but when it occurs, it presents with shortness of breath, dyspnea on exertion, a
nonproductive cough, and hypoxemia, associated with interstitial infiltrates on chest radiograph.
Neurologic Disease
CMV neurological disease causes dementia, ventriculoencephalitis, or ascending polyradiculomyelopathy.
CMV viremia
CMV viremia can be detected by PCR, antigen assays, or blood culture and is generally detected in end-organ
disease, but viremia also might be present in the absence of end-organ disease. The presence of serum antibodies
to CMV is not diagnostically useful.
CMV retinitis
The diagnosis of CMV retinitis is generally made on the basis of recognition of characteristic retinal changes
observed on fundoscopic examination by an experienced ophthalmologist.
CMV colitis
The demonstration of mucosal ulcerations on endoscopic examination combined with colonoscopic or rectal biopsy
with histopathological demonstration of characteristic intranuclear and intracytoplasmic inclusions are required
for the diagnosis of CMV colitis.
CMV esophagitis
The diagnosis of CMV esophagitis is established by the presence of extensive large, shallow ulcers of the distal
esophagus and biopsy evidence of intranuclear inclusion bodies in the endothelial cells with an inflammatory
reaction at the edge of the ulcer.
CMV pneumonitis
Diagnosis of CMV pneumonitis should be made in the setting of pulmonary interstitial infiltrates and
identification of multiple CMV inclusion bodies in lung tissue, and the absence of other pathogens that are more
commonly associated with pneumonitis in this population.
CMV neurologic disease
CMV neurologic disease is diagnosed on the basis of a compatible clinical syndrome and the presence of CMV in
cerebrospinal fluid or brain tissue.
CMV retinitis
The choice of initial therapy for CMV retinitis should be individualized. Oral valganciclovir, intravenous
ganciclovir, intravenous ganciclovir followed by oral valganciclovir, intravenous foscarnet, intravenous
cidofovir, and the ganciclovir intraocular implant coupled with valganciclovir are all effective treatments for
CMV retinitis.
CMV colitis or esophagitis
The choice of initial therapy for CMV colitis or esophagitis is intravenous ganciclovir or foscarnet.
CMV neurologic disease
For neurological disease, initiating therapy promptly is critical for an optimal clinical response. Combination
treatment with ganciclovir and foscarnet might be preferred as initial therapy.
Although drug resistance might be responsible for some episodes of relapse, early relapse is most often caused by
the limited intraocular penetration of systemically administered drugs. Because it results in greater drug
levels in the eye, the placement of a ganciclovir implant in a patient who has relapsed while receiving systemic
treatment (IV ganciclovir or oral valganciclovir) is generally recommended.
After induction therapy, secondary prophylaxis is recommended for life, unless immune reconstitution occurs as a
result of ART. Regimens demonstrated to be effective for chronic suppression include parenteral or oral
ganciclovir, parenteral foscarnet, combined parenteral ganciclovir and foscarnet, parenteral foscarnet,
parenteral cidofovir and (for retinitis only) ganciclovir administration through intraocular implant or
repetitive intravenous injections of fomivirsen. FDA has approved oral valganciclovir for both acute induction
therapy and for maintenance therapy.
1. Centers for Disease Control and Prevention. Treating opportunistic infections among
HIV-infected adults and adolescents: recommendations from CDC, the National Institutes for Health, and the HIV
Medicine Association / Infectious Diseases Society of America. MMWR 2004; 53(No.RR-15)
2. World Health Organisation. Antiretroviral therapy for HIV infection in adults and adolescents in
resource-limited settings: Towards universal access. 2006 revision.
http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf Accessed 15,th June 2006.
3. API Consensus guidelines for use of antiretroviral therapy in adults. JAPI 2006; 54:57-74.
4. AIDS. Devita VT, Hellman S and Rosenberg SA, Eds. Lippincott-Raven, 1997.
5. AIDS Therapy. Dolin R, Masur H and Saag MS, Eds. Churchill-Livingstone 2003.





















