Introduction of DPI
Asthma and Chronic Obstructive Pulmonary Disease (COPD) are both common conditions with an increasing prevalence worldwide.
Inhalation therapy is the main route of drug administration in patients with these conditions. Inhaled drug delivery systems are available into four principal categories: pressurised metered-dose inhalers (pMDls), dry powder inhalers (DPIs), breath actuated inhalers (BAIs) and nebulisers, each class with its unique strengths and weaknesses. The pMDI was first introduced 50 years ago and currently is the most commonly used device worldwide.
The development of DPI's has been motivated by the desire for alternatives to pMDls and to overcome the shortcomings of the pMDI. A DPI is a device that delivers medication to the lungs in the form of a dry powder. DPIs are easier to use, breath actuated, they do not require propellants and spacers and they eliminate the co-ordination problem and cold freon effect associated with the pMDls.
Most DPI formulations, as the name suggests, are powders consisting of a micronised drug blended with larger carrier particles, which enhance flow, reduce aggregation, and aid in dispersion.
Spinhaler (1969)
Rotahaler (1996)
The first capsule containing DPI appeared in the market in 1969 with the introduction of the Spinhaler (Fisons, UK) which delivered sodium cromoglycate. Cipla introduced the transparent Rotahaler in 1996, followed by a novel single-dose device, the Revolizer, and a multi-dose device, the Multihaler
Advantages and Disadvantages of DPIs
|
Advantages |
Disadvantages |
|
Easy to learn, simple to teach & Understand |
Dependence on patient's inspiratory airflow. With poor or improper inhalation, it is possible to get little or no drug into the lungs |
|
Compact & Portable |
Inconsistent efforts by patient could result in substantial variability between doses |
|
Little or no patient co-ordination required |
All DPIs are potentially vulnerable to humidity & moisture |
|
Multiple drugs can be administered from the same device (in case of single-dose DPIs) |
More expensive than other forms of inhalation devices |
|
No propellants, hence no cold freon effect and environment friendly |
May not be appropriate for emergency situations or during acute exacerbations |
|
No need of spacers |
Patients may need to inhale multiple times with single-dose DPIs |
DPI Formulation
Most DPIs consists of a Drug and Excipients
DPI FORMULATION = DRUG + EXCIPIENTS
Drug /Active Pharmaceutical Ingredient (API)
There are wide range of physio-chemical properties that make a drug suitable for pulmonary absorption (compared to orally administered drugs); there is nonetheless one critical requirement a drug must meet to qualify for respiratory delivery; this requirement is potency. Current inhalation devices limit the quantity of drug that can be delivered to the lungs in a single dose to a few milligrams. Thus, in order to be considered for inhalation therapy, drugs need to be therapeutically effective in the microgram or milligram range. (β2-adrenergic agonists, corticosteroids and anti-cholinergics are primarily used to treat obstructive airway diseases. Unlike systemically active drugs, the above 3 classes of drugs need not be absorbed into the circulation to exert their pharmacologic activity.
Excipients
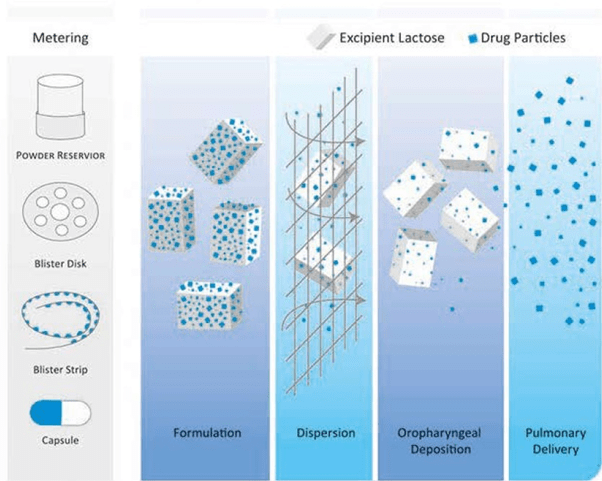
The emitted dose from an inhaler must contain drug particles <5µm to enable penetration into the airways. However, it is not possible to formulate DPIs to contain particles that are so small because the formulation will have poor powder flow properties. This would cause erratic inhaler filling during manufacture and inconsistent dose metering during patient use. Poor powder flow is thus improved by adding an excipient (usually lactose) to the drug.
In DPI formulations, excipients function first and foremost as carrier particles. Excipients are also used to enhance the physical or chemical stability of the active pharmaceutical ingredient. Usually, no more than a few milligrams of the drug need to be delivered, and excipients provide bulk, which improves handling, dispensing, and metering of the drug and reduce drug cohesiveness. Excipients can makes up over 99% of the product by weight, making them crucial determinants of overall DPI performance. Currently, lactose is the most widely used excipient in the marketed DPIs.
Lactose is an excipient of choice in DPIs due to following reasons:
- Established safety and stability profile.
- Widely available and inexpensive.
- Highly crystalline and has smooth surfaces.
- Satisfactory flow properties desirable for a DPI carrier particle.
- Less hygroscopic than other sugars.
Rotacaps which are to be used with single dose DPIs contains 15-25 mg of lactose; Multidose DPIs such as accuhaler and the multihaler contains 10-12.5 mg of lactose; whereas some reservoir DPIs contains 10-12.5 mg of lactose. Some reservoir devices in the market do not contain any lactose.
Particle Size of a DPI Formulation
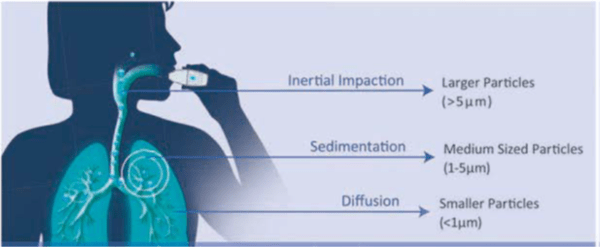
Particle size is the single most important design variable of a DPI formulation. It is expressed in terms of aerodynamic diameter. The aerodynamic diameter is commonly applied to inhaled drugs to predict where in the respiratory tract such particles will deposit. It is the most appropriate measure of aerosol particle size, because it relates to particle dynamic behaviour and describes the main mechanisms of aerosol deposition; both gravitational settling and inertial impaction depend on aerodynamic diameter. Upon entering the oral cavity, drug particles deposit by impaction, sedimentation and brownian motion depending on their size.
Aerodynamic Diameter
Airborne particles have a variety of shapes and sizes, so to understand their properties, the aerodynamic diameter is used. The aerodynamic diameter of an irregularly shaped particle is defined as the diameter of the spherical particle with a density of 1000 kg/m' that has the same settling velocity as the irregular particle.
|
Particle Size (microns) |
Regional Deposition |
Efficacy |
Safety |
|
>5 |
Mouth, oesophageal region |
No clinical effect |
Absorption from GI tract if swallowed |
|
1-5 |
Upper central airways |
Clinical effect |
Subsequent absorption from lung |
|
<1 |
Peripheral airway /alveoli |
Some local clinical effect |
High systemic absorption |
- Particles > 5 µm are most likely to deposit by impaction in the oropharynx and be swallowed. Lactose particles deposit in the oropharynx and mouth as they have particle size of > 5 µm (Table 2).
- Particles that are < 5 µm have the greatest potential for deposition in the lungs. These particles of 1-5 µm size usually deposit by sedimentation or gravity. The proportion of particles within an aerosol that are < 5 µm is often referred to as the fine-particle fraction (FPF), or the fine particle dose (FPD) if expressed as an absolute mass of drug in particles < 5 µm (Figure 1).
- Most particles of 0.1 - 1 µm diffuse by brownian motion and deposit when they collide with the airway wall. The longer the residence time in the smaller, peripheral airways, the greater the deposition from sedimentation and brownian motion processes. Inhaled particles that do not deposit are exhaled.
Summary
- In order to be considered for inhalation therapy, drugs need to be therapeutically effective in the microgram or milligram range.
- β-adrenergic agonists, corticosteroids and anti-cholinergics are primarily used to treat obstructive airway diseases
- The DPI formulation consists of micronised drug blended with larger carrier particles.
- In DPI formulations, excipients function first and foremost as carrier particles. Excipients are also used to enhance the physical or chemical stability of the active pharmaceutical ingredient.
- Currently, lactose is the most widely used excipient in the marketed DPIs.
- Upon entering the oral cavity, drug particles deposit by impaction, sedimentation and Brownian motion depending on their size.
- Drug particles that are between 1-5 µm have the greatest potential for deposition in the lungs. The proportion of particles within an aerosol that are <5 µm is often referred to as the fine-particle fraction (FPF), or the fine particle dose (FPD).
Working of DPI
As we know, DPIs consist of drug and excipient. DPIs employ the patient's inspiratory flow to work, i.e. the patient must provide the energy to disperse the drug from the device. When the patient activates the DPI and inhales, air flows through the device, which creates shear and turbulence; air is introduced into the powder bed and the static powder blend is fluidised. The fluidised powder is then passed through a screen or mesh (incorporated within the device), which de-agglomerates the particles. There, the drug particles separate from the carrier particles and are carried deep into the lungs, while the larger carrier particles impact in the oro-pharynx and are cleared (Figure 2). Thus, deposition into the lungs is determined by the patient's variable inspiratory airflow. In several marketed DPIs, drug delivery to lungs ranges between 20% to 30% of the emitted dose.
Airflow Path through DPI
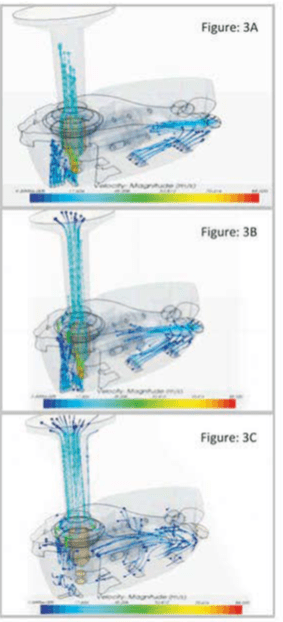
Computational fluid dynamics (CFD) have recently been used in the design of pharmaceutical inhalers. CFD provides valuable insight into certain aspects of inhaler performance. It is widely used in device design to determine airflow patterns and turbulence levels.
Below is the airflow patterns through the Revolizer. The Figures show the air flow path inside the Revolizer when patient inhales through the device. Figure 3A shows how air enters through various air vents and openings situated at the bottom of device as soon as the patient inhales through Revolizer. Figure 3B & 3C shows the direction of airflow through the Revolizer as patient continues to inhale.
According to the calculation of airflow rates using CFD, the velocity at the air inlets were in the range of 15-20 m/s.The outlet velocity followed linear airflow with a velocity calculated at 25 m/s. A pressure drop of 1.4 kPa was observed across the capsule chamber, creating a turbulence which in turn de-agglomerates the drug particles from the excipients. The pressure drop across the device was observed to be 4 kPa.
Reference.
Respiratory drug delivery 2014; 2: 401- 404
Summary
- DPIs employ the patient's inspiratory flow to work i.e. the patient must provide the energy to disperse the drug from the device.
- Drug deposition into the lungs is determined by the patient's variable inspiratory airflow.
- Drug delivery to lungs ranges between 20% to 30% of emitted dose for several marketed DPIs.
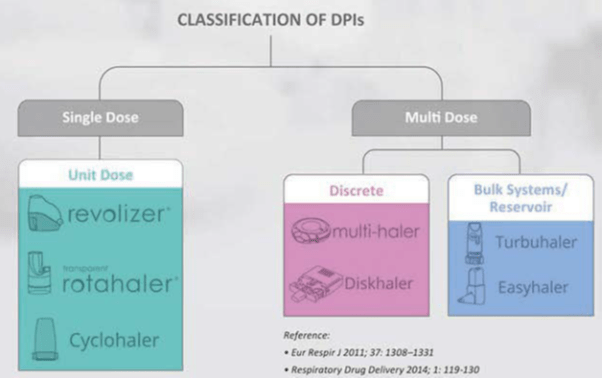
Classification of DPIs
“The ideal DPI is the one that delivers consistent and reliable doses, and is the one that patient trusts, finds easy to use and prefers over others.”
Single dose or unit-dose DPIs:
In single-dose or unit-dose devices, the drug is formulated as a micronised powder in a lactose excipient, supplied in individual Gelatin/Hydroxypropyl methyl cellulose (HPMC) capsules which must be inserted into the inhaler before use. The device has a mechanism for piercing the capsule.
The majority of single-dose DPI devices are primed by pressing, sliding, rotating or piercing to prepare the dose for fluidisation with tangential flow of air during patient inspiration. The fluidised powder is then passed through a screen (incorporated within the device), which de-agglomerates particles for lung delivery.
After use, the empty capsule must be removed from the inhaler before the next capsule can be placed in the device.
Multi-Dose Discrete DPIs
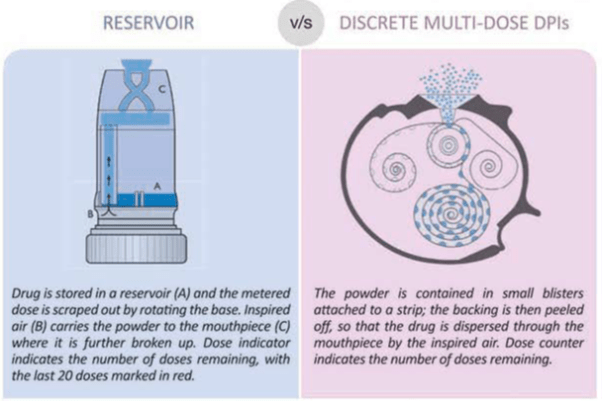
They contain preloaded individual doses in a blister strip. The sealed blisters offer a high degree of protection against humidity. The device is just needed to be twisted (or rotated) which pierces the blister and releases a drug dose for inhalation.
These also incorporate a dose counter, which enables the patient to monitor the number of doses remaining in the device. They have an integral outer case that serves to keep the device dust free and also resets the lever ready for the next dose.
The multi-dose DPI have an advantage over single-dose DPI as they contain preloaded doses and patients do not have to insert a capsule every time whenever the dose is required. Also, unlike single-dose DPI, they do not need to be cleaned periodically. However, their design is bigger and bulkier than other inhalers and the devices are more conspicuous when used in public.
Multi dose bulk systems / Reservoir
Multidose reservoir devices contain a bulk supply of drug from which individual doses are released with each actuation. The device consists of micronised drug which may be formulated with or without any additional lactose excipient. When the device is operated (usually by twisting or rotating the base), a measured dose is loaded for inhalation which gets released upon patients inhalation.
Reference
Med Eng Phys.2012, 34(4): 409.27, Prim Core Respir J. 2012; 22(2). 208-13
Note
Single dose and multi-unit dose inhalers are more effective than multi-dose reservoir devices as they ensure dose consistency and avoid the effects of moisture in the powder reservoir. Another advantage of unit-dose and multi-unit dose devices is the isolation of each dose, which facilitates storage stability. Some multi-dose reservoir types of devices lack dose uniformity during inhalation and stability of formulations, if they are not protected from environmental degradation. However, they are more complex due to the need to reload the device with a new cartridge/pack and patients (especially in the aged population) need appropriate education to operate the device.
Some patients also accidentally exhale into the DPI. This would be of greater significance with bulk reservoir devices, but less so for the unit dose devices. Exhaling into a DPI device presents two problems. First, exhaled air can blow the powder out of the chamber, making it unavailable for inhalation. Second, exhaled air is high in humidity, which can cause the carrier and/or drug to cake or agglomerate, reducing its ability to break up into individual respirable particles during inspiration. For reservoir DPI's, the powder remaining in the reservoir can, over time, might be affected by added humidity in the exhaled breath. Therefore, they are generally less favoured than single-dose and multi-unit dose designs.
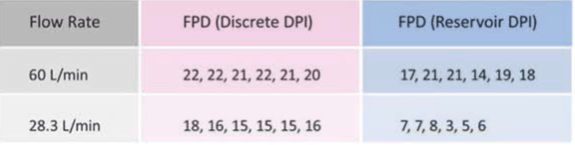
Fine particle dose (FPD) of delivered drug through discrete and reservoir DPIs:
A study compared the uniformity of fine particle doses emitted from a discrete and a reservoir DPI using the in-vitro method. The discrete multi-dose DPI emitted consistent fine particle doses at 60 L/min and also at low flow rate of 28.3L/min. On the other hand, at both flow rates there was a wide variability in the fine particle doses emitted from reservoir DPIs (Table 3).
Fine particle dose (FPD)
- The quantity of drug in the prescribed dose less than 5 µ in size.
- It gives an indirect estimation of the drug deposition in the lungs.
Summary
- DPIs are classified as single-dose (Revolizer, Rotahaler) and multi dose. Multi-dose DPIs are further classified as discrete (Multihaler, Accuhaler) and reservoir (Turbuhaler, Novolizer) depending upon whether they contain individual doses or bulk drug.
- In single-dose DPIs, the patient has to insert a capsule every time whenever the dose is required, whereas the multi-dose DPIs contain preloaded multiple doses.
- Single-dose and multi-unit dose DPIs are more effective than multi-dose reservoir devices as they ensure dose consistency, storage stability and avoid the effects of moisture in the powder reservoir.
- The discrete multi-dose DPIs emit a consistent fine particle dose at 60 L/min and also at a low flow rate of 28.3 L/ min. On the other hand, at both flow rates, there is a wide variability in the fine particle dose emitted from reservoir DPIs.
Steps for Using DPI Correctly
1. Check that the device is clean and the mouthpiece free of obstruction.
2. Load a dose into the device as directed. In case of single-dose devices, insert a capsule into the device and activate. In case of multi-dose or reservoir DPIs, the device needs to be primed by either rotating or sliding the base.
3. Breathe out fully through the mouth. (This action will empty the lung. Only when the patient exhales out completely, he/she can inhale to their full capacity). Position the mouthpiece between your teeth and close your lips tightly around it.
4. Keep your head straight and breathe in rapidly and deeply through the mouth. (This step is very important, because it is the patient's inhalation that causes the de-aggregation and evacuation of the drug in DPIs. With poor or improper inhalation, it is possible to get little or no drug into the lungs).
5. Remove the device from the mouth. Hold the breath for 10 seconds or as long as is comfortable. (Holding the breath allows settling of the inhaled particles into the lungs). Breathe out normally.
6. In case of a single-dose device, if some powder remains inside the capsule, repeat steps 3 to 5.
Rotahaler, Revolizer and Multihaler in Action
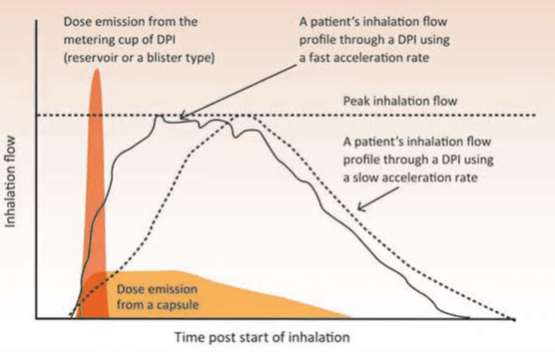
Relation between Patients Inhalation and dose emission from a DPI
Adopted from Eur Resplr 2011, 37: 1308-1331 (For presentation purpose only)
The above figure 4 shows two possible patient inhalation profiles. Both profiles attain the same peak inhalation flow, but one starts with a fast inhalation (i.e. fast acceleration rate) and the other gradually speeds up its flow (i.e. slow acceleration rate). It has been shown that the de-agglomeration of the particles takes place inside the device before the metered dose leaves the DPI and is increased if the acceleration is fast at the start of inhalation.
The de-aggregation of the metered dose in a DPI occurs during the first part of the inhalation manoeuvre and therefore the patient should be made aware that the forceful inhalation should be from the beginning. Failure to use a fast inhalation from the start through a DPI results in the emission of particles that are too big to be deposited in the lungs and so the dose is deposited in the oropharynx and subsequently swallowed. Thus, the Fine Particle Dose (FPD) will be greater and the Mass Median Aerodynamic Diameter (MMAD) smaller when the initial acceleration rate of the inhalation flow is fast. Hence patients should be instructed to inhale forcefully from the beginning and should not increase the speed of their inhalation gradually.
Superimposed onto the two inhalation profiles in the above figure are representations of when the dose is emitted from a capsule DPI and from a reservoir- or blister-type DPI. Clearly, the dose is emitted earlier during inhalation from the reservoir- or blister-type DPI, compared with the capsule DPI. For this reason, inhalation volume becomes important for patients using DP's with capsules, and patients should repeat the inhalation to ensure that they receive the full dose. Hence the patient should be instructed to inhale for as long as possible.
Many patients exhale through their DPIs, and this will introduce moisture into the formulation. This affects the powder flow which will cause problems with dose metering and de-aggregation. Depending on the internal design of the DPI, some will be affected more than others. Also, exhalation after the dose has been prepared for inhalation will blow the dose out through the inhalation channels of the DPI. The patient might therefore receive no or less dose during inhalation, so patients should be trained to exhale away from their inhaler.
Mass Median Aerodynamic Diameter (MMAD)
It is the diameter at which 50% of the particles of aerosol by mass are larger and 50% are smaller.
If the MMAD is 3 µm, then by weight
- 50% of the particles are > 3 µm
- 50% of the particles are < 3 µm
So MMAD gives a size range within which the drug particles in the formulation lies. A lower MMAD indicates deposition into peripheral airways.
Summary
- Patient should inhale forcefully through the DPI.
- Forceful inhalation should be from the beginning as the de-aggregation of the metered dose in a DPI occurs during the first part of the inhalation manoeuvre.
- Fine Particle Dose (FPD) is greater and the Mass Median Aerodynamic Diameter (MMAD) is smaller when the initial acceleration rate of the inhalation flow is fast.
- Patient should inhale for as long as possible particularly with the single-dose DPIs. This is because the dose is released gradually from the single-dose DPIs. One can also repeat the inhalation to ensure that they receive the full dose.
- Exhaling into the DPIs introduces moisture into the formulation and can also blow out the loaded dose. So patients should be trained to exhale away from their inhaler.
Common Patient Errors
Choosing the right inhaler device & following the correct technique is absolutely essential.
Despite the availability of high-quality drugs and devices, many patients still do not get the optimal benefits from them. A study estimates that, 55% of the asthmatics remain uncontrolled despite using standard asthma medications. One of the reason for this poor disease control is inappropriate use of the inhalers. Another study conducted in 4078 asthmatics revealed that, asthma control worsens as the number of mistakes in the inhaler technique increases.
Thus the inhaler devices play a very important role in determining optimal disease control. Choosing the right inhaler device and following the correct technique is absolutely essential.
Incorrect use of inhalers can lead to detrimental effects on disease management; nevertheless, studies have shown that patients do not comply with the individual steps of the inhalation maneouvre.
Whilst all inhaler errors have the potential to limit clinical efficacy, some errors are more important than others in this respect.
Inhaler errors are defined as critical if they substantially affect dose delivery to the lungs. Critical errors for all DPIs include lack of inhalation through the mouthpiece and blowing into the device before inhalation. Device-specific critical errors are lack of capsule insertion, lack of rotation, sliding or pressing to activate the device.
The most frequently observed errors with DPIs are
- Failure to exhale before inhaling
- Failure to forcefully and deeply inhale through the device &
- Failure to hold breath after inhalation
Other specific inhalation technique errors are as follows
- Incorrect inhaler position
- Incorrect dose metering
- Incorrect rotation, sliding or pressing to activate the device
- Not piercing the capsule
- Not releasing the piercing buttons
- Incorrect mouthpiece position (i.e. not positioning the mouthpiece correctly between the lips)
- Exhaling through the mouthpiece
- Failure to breathe out slowly after inhalation –
- Storage in high ambient humidity
Summary
- According to a study, 55% of the asthmatics remain uncontrolled despite using standard asthma medications. One of the reason for this poor disease control is inappropriate use of the inhalers.
- Asthma control worsens as the number of mistakes in the inhaler technique increases.
- Inhaler devices play a very important role in determining optimal disease control.
- Inhaler errors are defined as critical if they substantially affect dose delivery to the lung.
- The most frequently observed errors with DPIs are, failure to exhale before inhaling, failure to inhale forcefully and deeply through the DPI and failure to hold the breath after inhalation.
Important Points to Consider when Prescribing a DPI
Inspiratory flow rate and resistance of a device
"Inspiratory flow rate is the rate at which air is inhaled from a device".
"Resistance of a device refers to the effort the patient has to apply to inhale the drug from the device".
The formulation of a DPI is de-aggregated by turbulent energy that is created by the interaction of the patient's inhalation flow with the resistance inside the inhaler.
The turbulent energy is represented by a pressure change inside each DPI during the inhalation manoeuvre.
The turbulent energy inside a DPI during an inhalation is the product of the inhalation flow and the resistance of the device.
For low-resistance device, the inhalation flow through the device will be higher. On the other hand, in case of high resistance devices, the inhalation flow through the device will be lower.
For in-vitro evaluation of the DPIs, the resistance of a DPI can be classified with respect to the inhalation flow required to produce a pressure drop of 4 kPa. This value was chosen because it is the one recommended by pharmacopoeias and regulatory bodies for the in-vitro characterisation of the dose emitted from a DPI. A device that is characterised as having a low resistance requires an inspiratory flow of > 90 I /min to produce this pressure drop. A medium-resistance device requires 60-90 I/min. A medium/high resistance device requires 50-60 I /min and a high-resistance device requires <50 I /min.
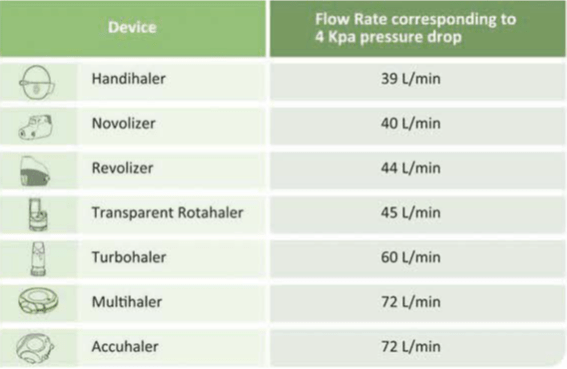
Inspiratory flow rates (approximately) required by some DPI's:
References:
Eur Respir 12011; 37 1308-1331
Respiratory Drug Delivery 2014; 2: 401.404
International Journal of COPD 2011:6 353-363
Multidiscip Respir Med. 2015 (den: 10.1186/s40248-015-0012-5)
The In-Check DIAL is an inspiratory flow meter that is used to assess the inspiratory flow of the patient (Figure 5). It can be used to measure a patient's inspiratory flow rate across a range of resistances matched to the inhaler devices. This training tool enables clinicians to demonstrate to patients that they can generate a sufficient flow rate for their prescribed device, and particularly to identify patients with a very low inspiratory flow rate (such as young children and those with severe, fixed airways obstruction) who may not be able to generate sufficient flow and for whom the device may be unsuitable.
Thus healthcare professionals can use the In-Check DIAL as a useful tool to train patients on correct inhalation with devices.
*http://www.clement-clarke.com/ProductInfo/InhalerTechniqueTraining/InCheckDIALaspx (For presentation purpose only)
Are DPIs of a Particular Resistance Better than Others?
Normally it is known that, DPIs with low resistance are easier to use, because the flow rates through these DPIs will be higher. But research indicates that, even the high-resistance devices are as good as low-resistance ones.
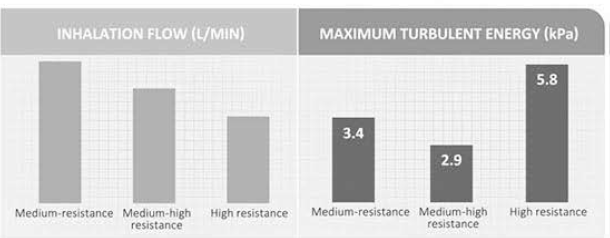
Let's take one example. A study was conducted in COPD patients using three DPIs with different resistance:
- Medium-resistance DPI
- Medium-high resistance DPI
- High resistance DPI
It was found that inhalation flows through a medium, medium-high and high resistance DPI's were in the expected order, but the maximum turbulent energy inside each device during inhalation was 3.4kPa, 2.9kPa and 5.8kPa, respectively (Figure 6).
Thus, although the flow through the high-resistance DPI was the lowest, the turbulent energy available to de-aggregate the dose was the highest. "These energies may explain the different flow-dependent dose emissions of these DPIs and why DPIs with a high resistance tend to provide greater lung deposition with less variability than those with a lower resistance".
The resultant turbulent energy generated from low inhalation flow in high-resistance DPIs was sufficient to de-aggregate the dose and highlights the fact that each type of DPI is unique.
Can DPI's be used during Exacerbations?
During exacerbations the inspiratory effort decreases; this could translate into a reduced dose emission from a DPI. This concern is also directed to patients with COPD and young children with asthma because of their reduced inspiratory effort. Thus reduced dose delivery from all DPIs should be taken into consideration during exacerbations.
However a study conducted on 153 children aged above 5 years with mild-to-moderate acute exacerbations of asthma showed that they could effectively use a Rotahaler. The study concluded that a Rotahaler and a pMDI + spacer have equal efficacy in delivering salbutamol in mild-to-moderate acute exacerbations of asthma in children. Another study revealed that even severe asthmatics can achieve a high inspiratory flow rate but for a shorter time. This was found to be sufficient to activate most of the available DPIs.
Which Age Group Patients can use DPIs?
As we know, the decisive factor for therapy success with DPIs is the generation of sufficient inspiratory flow to trigger the dosage and de-agglomerate the powder. The patient needs to breathe forcefully and deeply to disperse the powder in the DPI. Most patients can achieve adequate flows through their DPI. However, few patients are unable to breathe forcefully through their DPIs, resulting in poor drug release and low pulmonary deposition.
This is particularly the case for some younger children, older patients (aged above 60 years), and some patients with severe airflow limitations. However, as stated earlier, children aged above 5 years with mild-to-moderate acute exacerbations of asthma could effectively use a Rotahaler.
In general, DPIs can be easily used by any patient, aged 4 years and above. (If the patient is confident and prefers it over other devices).
Summary
- Inspiratory flow rate is the rate at which air is inhaled from a device.
- Resistance of a device refers to the effort the patient has to apply to inhale the drug from the device.
- For low-resistance devices, the inhalation flow through the device will be higher. On the other hand, in case of high resistance devices, the inhalation flow through the device will be lower.
- In-Check Dial is a useful tool to train patients on correct inhalation with devices. This training tool enables clinicians to assess whether patients can generate a sufficient flow rate for their prescribed device.
- Each type of DPI is unique as each DPI has a different flow-dependent dose emission.
- DPIs with low resistance are easier to use, because the flow rates through those DPIs will be higher. But research indicates that, even the high-resistance devices are as good as the low resistance ones.
- The resultant turbulent energy generated from low inhalation flow in a high-resistance DPI is sufficient to de-aggregate the dose.
- DPIs with a high resistance tend to provide greater lung deposition with less variability than those with a lower resistance.
- During exacerbations, the inspiratory effort decreases; this could translate into a reduced dose emission from a DPI. However a study has shown that children (aged above 5 years) with mild-to-moderate exacerbations could effectively use a single-dose DPI (Rotahaler)
- In general, DPIs can be easily used by any patient, aged 4 years and above.
Clinical Efficacy
DPIs: As effective as pMDI + Spacer in Asthmatic Children
A study was conducted to compare the response to salbutamol inhalation delivered by metered dose inhaler with a 750 ml spacer with valve (Cipla Ltd) versus a Rotahaler (Cipla Ltd) in 153 children in the age group of 5-15 years presenting with mild or moderate acute exacerbations of asthma. The children were randomised to receive 400µg salbutamol by either a pMDI with spacer or a Rotahaler.
After receiving treatment, the PEFR improved by about 11% in both the groups. The oxygen saturation increased by 2% in both the groups. Within each group, the improvement in PEFR, Sa02, wheeze and accessory muscle score after the treatment was statistically significant. In both the groups the children co-operated equally well.
The authors concluded that metered dose inhaler with spacer and dry powder inhaler have equal efficacy in therapy of bronchial asthma in children.
Indian Pediatrics 2004; 41:15-20
Comparison of pMDI + Spacer, DPI and Nebulizer
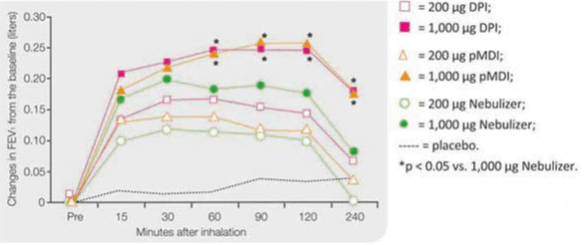
A study was conducted to compare the bronchodilator effects of salbutamol delivered via three different devices: a dry-powder inhaler (Rotahaler), pressurised metered-dose inhaler (pMDI) with a large-volume spacer and a jet nebulizer (NEB) in 10 stable COPD patients.
With the 200 µg dose, only DPI produced a small but greater response in maximum FEV, and in the area under the time response curve (AUC- FEV,:) compared with placebo. With the 1,000 µg dose, the DPI and the pMDI produced equally greater improvements in both maximum FEV,: and AUC—FEV, than the nebulizer (Figure 7).
Thus to conclude an equal bronchodilating effect was obtained using either a DPI or a pMDI with a spacer device, whereas the nebulizer was less effective when the same dose was administered.
Respirartion 1999; 66: 119-123
Multidose Single-Unit DPI: As Effective As pMDI
A multicenter, randomised, double-blind, 12 week study was designed to evaluate the efficacy of salmeterol/fluticasone combination (SFC) administered via multidose dry-powder inhaler and pMDI in 247 COPD patients (aged > 40 years).
At endpoint, pre-dose FEV,, FVC, PEF, and salbutamol use were similar between the two devices. Thus it was found that SFC delivered via a multidose unit dry powder inhaler was as effective as when delivered via a pMDI.
Open Respir Med J 2010; 21(4): 86-91
Comparison of Multidose DPI with the Breath Actuated Inhaler (BAI)
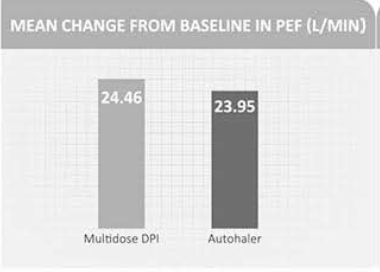
An 8-week open study in 209 moderate-to-severe asthma patients compared multi-dose DPI with the BAI (Autohaler). Patients were randomised to Beclomethasone 800 mcg autohaler or Budesonide 1,600 mcg multi-dose DPI.
As seen from the graph, the mean change in AM PEF was equivalent for the two groups (Figure 8).
Also there were no significant differences in the mean change from baseline in FEV, or beta2-agonist use in patients using either the BAI or DPI.
It was concluded that the BAI and a multidose dry powder inhaler were found to be similar in efficacy and safety in moderate-to severe asthmatics.
Respiration 2001; 68(5): 517-26.
Summary
- Metered dose inhaler with spacer and dry powder inhaler (Rotahaler) were found to have equal efficacy in therapy of bronchial asthma in children aged 5-15 years.
- A study conducted in COPD patients showed equal bronchodilation effect with either a DPI (Rotahaler) or a pMDI + spacer.
- Salmeterol Fluticasone Combination delivered via multidose unit dry powder inhaler was found to be as effective as pMDI.
- The BAI and a Multi-dose DPI were found to be similar in efficacy and safety in moderate-to-severe asthmatics.
Patient Perspective
The many marketed dry powder inhalers have different designs and method of operations. Studies using different dry powder inhalers have confirmed that different lung deposition patterns are observed with different DPIs. Furthermore, there may be considerable individual variability in lung deposition. The ideal inhaler does not yet exist. Different dry powder inhalers show some but not all features of the ideal inhaler; hence, patients may prefer some aspects of one inhaler while favouring a different inhaler for other features. The individual balance of features will govern the overall preference for one inhaler over others. Evidence suggests that patients cannot use all dry powder inhalers equally well.
Studies have also shown that patients do not comply with the individual steps of the inhalation maneouvre. Incorrect use of inhalers can lead to detrimental effects on asthma management. It has been found that when patients were given instructions and training on the usage of devices, their handling of the devices improved and the errors decreased.
In a study done in COPD patients using various dry powder inhalers, it was found that the multidose devices were preferred by the patients over single-dose devices. Another study comparing DPIs with pMDls concluded that DPI were the most preferred and easiest form to use but they are also expensive. The study concluded that if DPIs are prescribed, 89.5% of patients would definitely comply with the prescriptions. DPIs may be the best choice when long-term compliance is considered.
Summary
The many marketed DPIs have different designs and method of operations. There is a considerable individual variability in lung deposition with different DPIs. The ideal inhaler does not yet exist.
Giving patients instruction and training on the usage of devices, improves the handling and reduces the errors.
A study done in COPD patients showed that patients prefer multidose devices over single-dose devices.
Another study concluded that patients prefer DPIs over pMDls. However they are more expensive than the pMDls.
DPIs may be the best choice when long-term compliance is considered.
Cipla’s Range of DPIs
Transparent Rotahaler
The Rotahaler introduced in 1996, brought about a revolution in the field of inhalation therapy in India. The Rotahaler heralded the era of patient-friendly inhalation therapy and led to wider acceptance of the benefits of inhalation therapy. Since its introduction in 1990s, millions of asthmatics have used the Rotahaler with dramatic results.
The features of Rotahaler are as follows
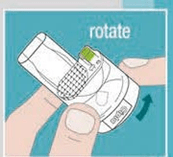
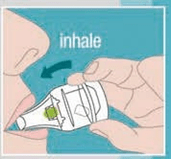
- Simple to use: Just require 3 steps 1. Insert Capsule 2. Rotate 3. Inhale Transparent: Gives visual feedback to the patient.
- Rattling sound of capsule: Gives audio feedback
- Material: Non-toxic. tough and scratch-resistant polycarbonate material.
- Shape: Designed aerodynamically to provide good turbulence for effective dosing.
- Shaft: It separates the capsule's cap and body when the device is twisted
- Round-bottom base: Enhances the turbulence required for effective dosing.
- Whistle-shaped mouthpiece: Provides better grip of the device during inhalation.
- Mesh: Ensures that the fragments of the capsule will not be inhaled.
- Robust: Well-equipped to survive the rigors of patient handling.
- Resistance: Medium-resistant device - gets activated at 45 L/min.
- Stability: Extensive stability studies carried out under high temperature and high humidity (25°C at 60% RH & 40°C at 75% RH) have shown that the Rotahaler and the Rotacaps remain stable even in extreme conditions.
- One device for all the medication: All the medications can be administered through just one simple device.
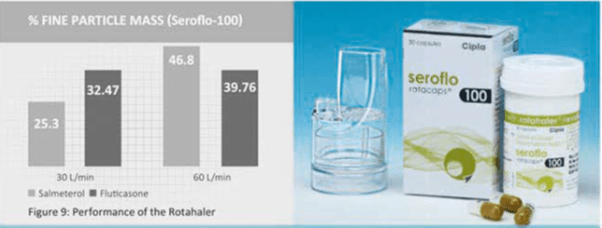
Performance of the Rotahaler
The percent fine particle mass of salmeteroI and fluticasone in Seroflo-100 rotacaps used with the Rotahaler has been studied using Multistage liquid Impinger Apparatus at flow rates of 30 L/min and 60 L/min. The Rotahaler showed an optimal drug deposition of both the drugs at 60 L/min and even at low flow rates of 30 L (Figure 9).
Rotahaler - Backed by strong research
The Rotahaler has been extensively studied. The key outcomes from these studies are given below:
Rotahaler: High Patient Preference
References
1.Current Allergy and Clinical Immunology 2003; 16(3): 118
2. American College of Chest Physicians, South Indian Chapter, Chennai, August 2000
Summary
- The Rotahaler introduced in 1996 is the World's 1'' transparent DPI.
- It revolutionised the inhalation therapy in India.
- The device just requires 3 steps to use — Insert Rotacaps, Rotate and Inhale.
- It gives patients audio, visual and taste feedback which confirms the delivery of the dose.
- It produces an optimal lung deposition at 60 L/min and even at low flow rate of 30 L/min.
- The Rotahaler is backed by 7 clinical studies that reinforce its role in asthma and COPD.
- The Rotahaler was perceived as easy to use by 98% of patients while around 94% preferred it over a pMDI+spacer.
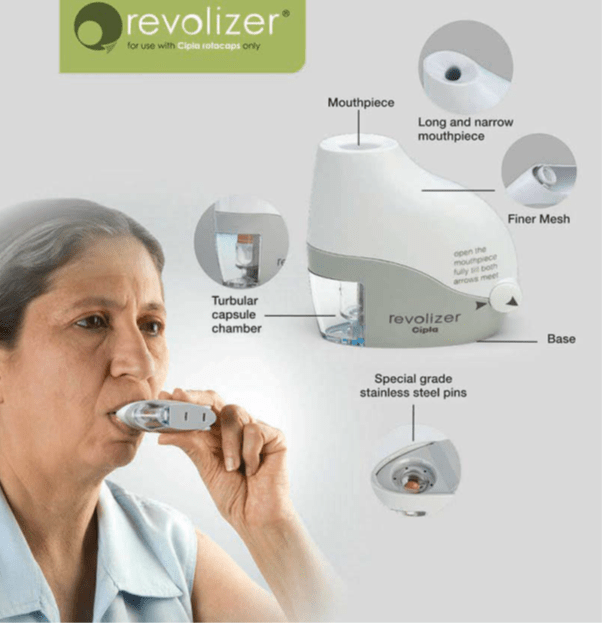
Revolizer
The Revolizer is a novel Dry Powder Inhaler (DPI) device, which is the result of a design and development programme to address the critical requirements of an ideal inhalation device. It is probably the easiest device to use. The patient simply opens the Revolizer, inserts the Rotacap, shuts the device and inhales.
It provides accurate dosing and optimal lung deposition even at low inspiratory flow rates combined with ease of use. It provides an improved respirable fraction while adding style and modern design to give patients a revolution in inhalation therapy.
The features of the Revolizer are as follows
- Easy to use: Open the device, inserts the Rotacaps, shuts the device and inhale.
- Sleek design, visually appealing and robust.
- Long and narrow mouthpiece: Leads to laminar airflow, creates more turbulence of the capsule and thus facilitates easier evacuation.
- Tubular Capsule Chamber: Helps de-aggregation of the drug and lactose and results in easier evacuation.
- Transparent Medication Chamber: Enables the patient to see that the dose has been inhaled.
- Finer Mesh: Filters even the smallest fragment of the capsule, if it breaks.
- Spike assembly: Made up of 304 stainless steel which pierces two holes in the capsule for inhalation.
- Resistance: Medium-resistant device - gets activated at 44 L/min.
- Vibration sound of the capsule: Gives audio feedback to the patient.
- Baffle: Ensures maximum drug delivery to the lungs.
- Convenient: Can be held in any position for the powder to evacuate from the capsule.
- One device for all medication: All the medications can be administered through just one simple and stylish device.
Performance of the Revolizer
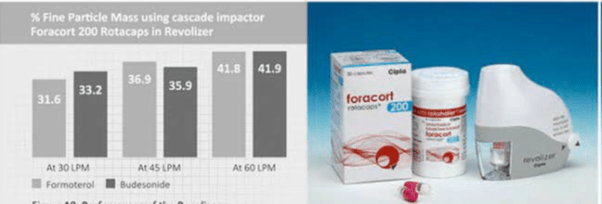
The percent fine particle mass of formoterol and budesonide in Foracort rotacaps with Revolizer has been studied using the Cascade Impactor at various flow rates of 30 L/min, 45 L/min and 60 L/min. The Revolizer showed an optimal drug deposition of both the drugs at 60 L/min and even at low flow rates of 30 L/min (figure 10).
Experience with the Revolizer in Indian Patients
A study was conducted to assess the confidence, usability, preference and satisfaction on the use of the Revolizer in healthy volunteers and in subjects with mild asthma or COPD.
- i prefer: Indian patent’s preference based on effectiveness and satisfaction with Revolizer
- i-prefer was the 1st study which was conducted on an indigenously developed device (Revolizer) in Indian patients.
- This study was accepted and presented at European Respiratory Society (ERS), 2010 - Barcelona.
- This study was also published in the Lung India journal. (Lung India 2014; 31(4): 366-374)
Results
Other major results of the i-prefer study
- Patients took only 3 minutes to use the Revolizer correctly for 3 consecutive attempts.
- .On an average patients made 2 attempts to learn the correct use of Revolizer.
- 92% of patients found it was easy to keep the Revolizer clean.
Conclusion
The results of the i-prefer study show that the Revolizer is easy to use and remember. The participants preferred the device and showed overall satisfaction. Thus, the Revolizer represents a reasonable alternative to other available DPIs.
Summary
- The Revolizer is a novel Dry Powder Inhaler (DPI) device, which is the result of a design and development programme to address the critical requirements of an ideal inhalation device.
- It provides an improved respirable fraction while adding style and modern design to give patients a revolution in inhalation therapy.
- The device just requires 3 steps to use - Insert Rotacaps, Shut the device and Inhale. Revolizer produces an optimal lung deposition at 60 L/min and even at low flow rates of 30L/min.
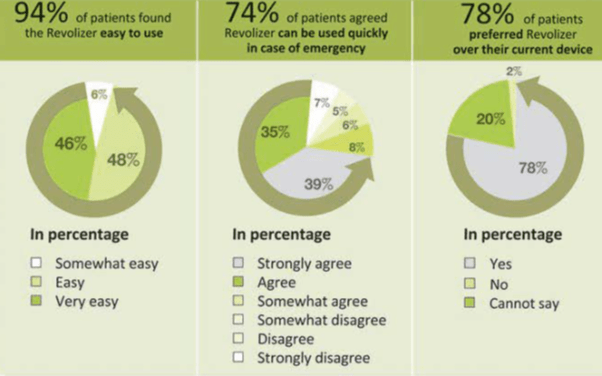
- The Revolizer was found to be easy to use by 94% of patients.
- 74% patients agreed that Revolizer can be used quickly in case of emergency.
- 78% patients preferred Revolizer over their current device.
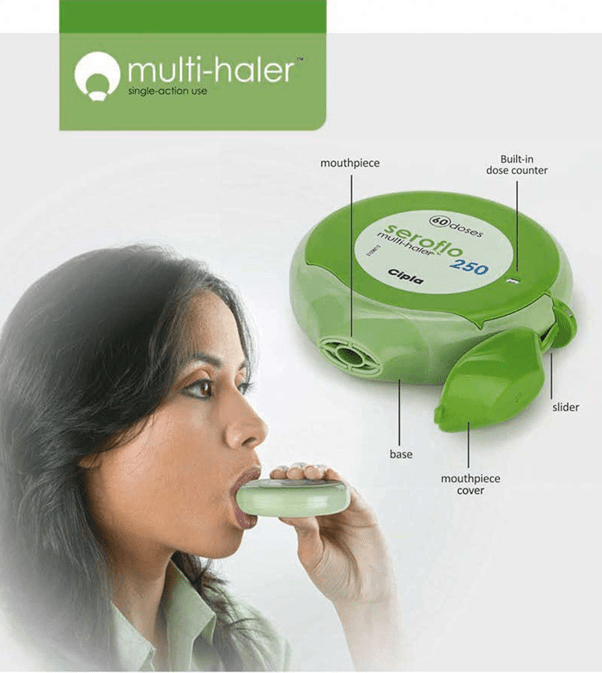
Multihaler
The Multihaler is a multi-dose dry powder inhaler (DPI), which disperses individual, pre-metered doses from a blister and it needs just a single step for use. A simple action of sliding the mouthpiece cover punctures a blister to release the drug powder, which is then inhaled through the mouthpiece. The reproducibility of the pre-measured dose is assured during the formulation, dose-filling and the device assembly process.
The features of the Multihaler are as follows:
- Simple to use: Open the mouthpiece cover, slide and inhale
- Easy to teach and learn.
- Ready to use: No need to load individual doses
- Taste feedback: Leaves a sweet taste after inhalation.
- Built-in dose counter: Confirms dose taken and also indicates the number of doses left.
- Convenient to carry, Robust design.
- Performance not affected by humidity or temperature
Principles of Operation
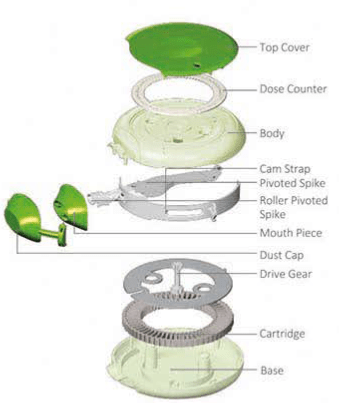
The image shows internal parts of the Multihaler. The drug doses are present in the cartridge which contains 60 doses filled in the cavities. The doses are covered with a uniform layer of aluminium foil (Figure 11).
During use, when the patient slides the slider, cam strap present inside moves in synchrony. At the same time, the pivoted spike moves and comes over the loaded dose. As the patient moves the slider further, the spike punctures the aluminium foil of the loaded dose. The Multihaler is now ready for use.
The patient now inhales through the mouthpiece which fluidises the powder bed. The drug is de-aggregated and then evacuated through the mesh, and finally into the mouthpiece.
After inhaling the dose, the patient moves the slider back. As the result the cam strap and spike comes back to their original position. The dose counter decreases by 1 unit and shows the number of doses remaining.
Performance of the Multihaler
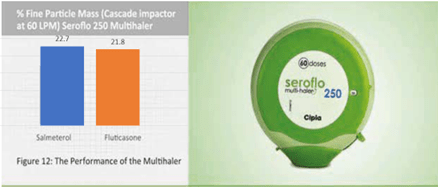
The percent fine particle mass of salmeterol and fluticasone in the Seroflo 250 Multihaler (60 doses) has been studied using a Cascade Impactor at 60 L/min Seroflo 250 Multihaler (60 doses) showed an optimal drug deposition of both the drugs (figure 12).
Multihaler — Research Studies
The outcomes of the studies were as follows
- In an open-label, prospective study carried out in 71 asthmatics it was found that 84% of patients rated Multihaler easier to use as compared to their previous inhalers. It was concluded that the Multihaler is effective, easy to operate and has good acceptability in patients with persistent asthma.(Presented at the European Respiratory Society (ERS), 2007 - Stockholm. Sweden)
- In another open-label, prospective, 2 visit study done on 103 (equal no of asthmatics and healthy subjects) patients, it was found that the Multihater is easy to operate and acceptable with a high level of confidence (Presented at the European Respiratory Society (ERS),2007 – Stockholm, Sweden
Summary
- The Multihaler is a multi-dose dry powder inhaler, which contains individual, pre-metered doses in a blister.
- To use the device, one just needs to open the cap, slide and inhale.
- It has a built-in dose counter which confirms the dose taken and also indicates the number of doses left.
- The performance of the Multihaler is not affected by humidity or temperature.
- It shows optimal lung deposition at 60 L/min.
Conclusion
Inhalation therapy is extensively used for the treatment of chronic respiratory diseases such as asthma and COPD.
It was only 60 years ago that the first portable pMDI containing a bronchodilator was introduced. For about 46 years, DPIs have been available as an alternative to pMDI and have gained a substantial share of the market due to their inherent advantages.
The pMDIs constitute the first inhalation device which was introduced in the 1950's. DPIs which were introduced in the late 1970's soon became popular. Currently there is a move away from the traditional pMDls towards newer, more sophisticated DPIs reflecting the increased research into powder formulations and innovations in particle engineering. Capsule-based devices in particular, offer many benefits to patients in terms of precise dosing, ease of use and dose visibility. The devices are hygienic to use and simple to clean and are presented in attractive designs.
Presently, different types of dry powder inhalers are available. These have different output and handling characteristics. Therefore, it is important to select the best DPI for any given patient, based on aerosol delivery, ease of handling and patient preference because these factors directly affect treatment compliance and therefore disease control. The prescriber must therefore ensure that the selection of a delivery device is appropriate for the individual patient needs.
An ideal dry powder inhaler should be easy to use, easy to remember and teach, effective even at low inspiratory flow rate, and preferred by all types and age groups of patients. No matter how good the device is, it is absolutely essential that the patient should use it with the appropriate technique. The healthcare professional should instruct patient in the proper technique of using the device. Training must be device specific and a placebo inhaler can be helpful.
Dry powder inhalers now have an established role in inhalation therapy. It is estimated that more than 113 million DPI's are dispensed worldwide in a year. In recent years, there has been a dramatic increase in the number of patent applications for new DPIs and it is anticipated that many more will be introduced in the future. As more DPIs become available with different designs and performance, clinicians need to evaluate the differences in various DPI's to make optimal therapeutic choices.
References
1. Lung India 2014;31(4):366-374
2. Respiratory Drug Delivery 2014; 2:401-404
3. Prim Care Resplr J 2012; 21(2): 208-13
4. Lancet 2011;377:1032-45
5. Eur Respir J 2011;37:1308-l331
6. Respr Med 2010; 104(9):1237-1245
7. Open Respir Med J 2010; 21(4); 86-91
8. Respir Care 2008;53(6):699 -723
9. lndian J Chest Dis Allied Sci 2008; 50:209-219
10. Respir Med 2008;102:10-19
11. Respir Med 2008; 102:593-604
12. lnt J Clin Pract 2007; 61(6):1022-1036
13. Respir Care 2005;50(9):1209-1227
14. Indian Pediatr 2001, 38(1): 24 8
15. Respiration 1999;66(2):119-23
16. Eur Respir J 1999; 14:525s
17. Respr Med 1998;92:105-110
18. Data on file, Cipla Ltd