Optimizing Inhaled Drug Delivery - Focus on Spacers
Introduction
The pressurized metered dose inhaler (pMDI) is the most widely used inhaler device in the world, with more than 500 million produced annually.9 Spacers are extensions to a pMDI, with a port at one end to which the pMDI is attached and a mask or mouthpiece fitted at the other end. They help in holding the medication for a few seconds after it has been released from the pMDI. The first pMDI in 1956 had a spacer.
History of Spacers
The original pMDI product, as described in 3M's 1956 sales brochure, had an elongated mouthpiece. The purpose of this design was to allow some time and space for the rapidly moving propellant droplets to evaporate. This reduced the impact of the drug at the back of the throat, possibly increasing lung deposition. In effect, this was the first pMDI spacer device. Some years later, a larger spacer device was used for the delivery of inhaled corticosteroids to children.
This was a chamber that had a volume capacity of about 1 litre. Subsequently, a range of such devices were developed and marketed. With greater understanding of the working of spacers, these have now been further modified to include non-static spacers.
Working of a Spacer
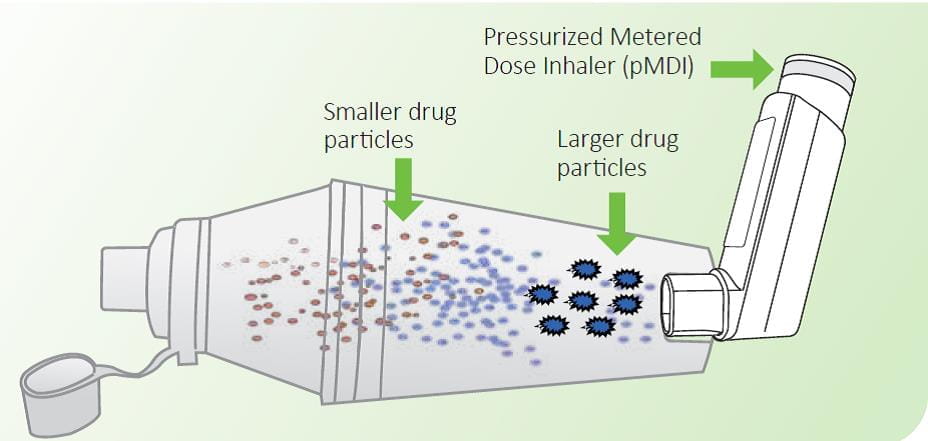
Spacers extend the mouthpiece of the inhaler and direct the cloud of medication towards the mouth. When the pMDI is fired, or actuated, the aerosol cloud produced from the spacer device is finer and slower moving than when released from a pMDI alone. This results in evaporation of the propellants and settling down of the larger particles in the spacer.1
This increases the amount of drug reaching the lower airways and limits the amount of particles in the mouth and throat, which in turn reduces the amount of drug absorbed systemically. At least 20% of the dose released from a pMDI will be delivered to the lungs via the spacer.
Using a spacer helps reduce the oropharyngeal deposition, thus reducing the local side effects associated with the use of high dose inhaled corticosteroids.1
Generally, patients using pMDIs with a spacer should be trained to inhale slowly (≤30 liters/minute) and to hold their breath after aerosol inhalation for at least 10 seconds.
Advantages of Spacer Devices
Making pMDIs Easier to Use
Nearly 80% of the patients fail to use the pMDI correctly, with the major problem being coordinating the actuation with inhalation. This is easily overcome by a spacer, which holds the medication for a short time till the patient inhales.2
Reducing Oro-pharyngeal Deposition
Normally, with a pMDI, close to 70–80% of the drug is deposited in the oro-pharynx. This is due to the speed with which the drug is emitted. When a spacer is used along with a pMDI, oro-pharyngeal deposition drops by more than 50% since most of the larger particles settle on the walls of the spacer. This reduces the likelihood of local as well as systemic side effects. Guidelines recommend the use of spacers in patients who are on high dose of steroids.3 The British Thoracic Society recommends the use of spacers in patients who are on a high dose of steroids (more than 1,000 mcg/day).
Improving Pulmonary Deposition
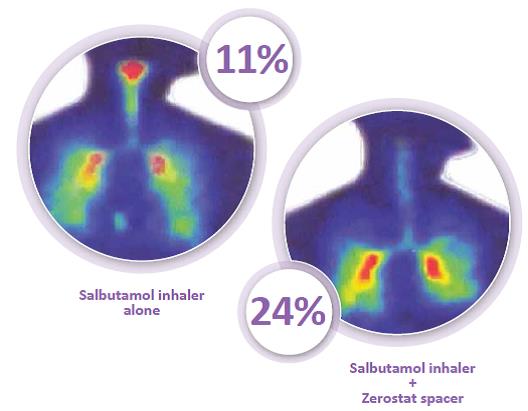
The spacer device helps to overcome the issue of coordination seen with pMDI and reduces the velocity of the aerosol spray as it passes through it.1 The propellant droplet size is reduced by evaporation. This results in increased lung deposition. For example, in a study conducted in Mumbai, the mean percentage of salbutamol deposited in the lungs was found to be 11% with the pMDI alone, but the deposition increased to almost 24% with the use of a Zerostat Spacer.
As an Alternative to Nebulizers in Acute Asthma Attacks
A systematic review of studies of treatment in children with acute exacerbations of asthma has found a pMDI with a spacer to be as effective as a nebulizer.4 This was also demonstrated in a study from New Delhi by Batra et al.5 Also, compared to nebulizers, pMDIs with spacers have the following benefits:
- Cause fewer side effects due to less drug dose used.
- Provide greater savings in cost.
- Require no electric power.
- In case of the patient being hospitalized, facilitates faster discharge from the emergency department.
Who should use Spacers?
- Children and the elderly. Patients with co-ordination problems
- Those who are prescribed high-dose inhaled steroids (more than 1,000 mcg/day)
- Patients with acute asthma requiring high-dose bronchodilators, as a substitute to nebulizers
- Patients who are prescribed anti-cholinergic drugs (to avoid the spray particles from reaching the eyes)
- Infants and small children by attaching a mask with the spacer3
Types of Spacers
As a class, Spacers are also known by other names, including “add-on devices” and “extension devices”.
Spacers can be classified into the following categories1:
Simple Tube Extensions
These are open tube spacers with no valve that simply distance the inhaler mouthpiece from the patient's oro-pharynx. Example: Zerostat Spacer.
Holding Chamber
Holding chambers share the properties of extension devices, but, in addition, they reduce the need for co-ordination between pMDI actuation and inhalation, and are generally easier for children and older patients to use than a conventional pMDI alone.
These have a “one-way valve” in the mouthpiece intended to hold the aerosol within the device until the patient's inhalation opens the valve. They provide additional space for the aerosol plume to develop. The presence of a valve in a spacer prevents the dilution of the drug dose in the spacer as the patient exhales into the device.
However, the pressure flow characteristics of the valve can play a significant role in modifying drug delivery.6 The valve that operates on inhalation and exhalation must function effectively over the entire pressure range likely to be encountered with use of the valved holding chambers. This requirement is unlikely to be a problem for adults. However, infants may be unable to generate sufficient pressure to open the valve. Hence, the valve should have low resistance so that it functions even at low inspiratory flow rates. Further, the valve should be robust, durable and easy to clean.
Spacers reduce the need for patients to co-ordinate inhalation with actuation of the pMDI, but to maximize the drug delivery period, inhalation should start as soon as possible after actuation of the inhaler. Delay between actuation and inhalation may reduce the amount of drug available to the patient, especially in case of static spacers.
Patients who are unable to hold their breath for 4 seconds should use a spacer with a one-way valve (holding chamber), allowing the patient to obtain a suitable dose of medication in three to four tidal breaths. Valved holding chambers allow the patient to breathe tidally from the reservoir of drug and are especially helpful for children. The availability of a face mask for use with holding chambers has markedly improved the opportunities for the treatment of very young children with asthma. Example: Zerostat VT, Minizerostat Spacer.
Factors Affecting Drug Delivery through Spacers
Spacer Volume
The cumbersome and bulky nature of the spacer has always been an impediment in its usage by patients. Hence, there is a need to balance the dimensions of a spacer for optimal aerosol delivery and clinical convenience. As the spacers have evolved, there has been a shift from the large-volume static spacers (750 ml) to smaller-volume anti-static spacers (300 - 150 ml), which are portable and easy to use. There has always been a debate whether the large-volume or the small volume spacers are ideal. Evidence supporting the effect of spacer size on clinical evidence is sparse. In one of the earlier studies supporting the development of more patient-friendly, small-volume VHC*, Corr et al, evaluated the fine particle output through spacers of varying sizes. Results of the study indicated that a tube 11 cms in length and 3.5 cms in diameter, delivered majority of respirable aerosol, measured using a cascade impactor. Any further increase in these dimensions failed to confer any significant benefit in terms of respirable dose. J Aerosol Sci. 1982; 13:1–7. Factors such as ease of use, pocketability, portability, patient satisfaction should be considered important while selecting spacers, as they will help in improving adherence to usage of spacers. There is no benefit of developing a spacer which shows a high in vitro performance in terms of respirable fraction, if the patient is not satisfied with it and does not use it.
“Engineers are likely to opt for the most efficient device in terms of aerosol output, clinicians have to take other factors into account viz, portability, ease of use to obtain optimum patient compliance”.6
The adults can use both small as well as large-volume spacers. However, the large volume spacers are bulky and difficult to carry.
Spacer Shape: Cone or Pear-shaped
Spacers are generally of a cone or pear shape. The closer the shape of the spacer to the shape of the aerosol plume discharged from the pMDI, the better it is. Pear-shaped spacers may be particularly effective in reducing particle loss within the device and the mouth, given their ability to collect the aerosol plume and allow the propellant to evaporate. Therefore, the ideal shape for a spacer is a cone or a pear shape.
A cylindrical spacer will cause more aerosol deposition onto the walls of the spacer, leading to reduced delivery of the drug dose to the patient.1
Electrostatic Charge: Non-static Spacer is the Best
Static charge plays an important role in the performance of the spacer. The surface of plastic spacer accumulates static charge. Aerosol particles from an MDI are rapidly deposited on the walls of the static spacer, rendering them unavailable for inhalation. This leads to a significant reduction in dose available for inhalation thus decreasing the delivery of drug to the lungs. This electrostatic charge varies in a random manner, depending upon the condition of the spacer–plastic spacers initially have a strong electrostatic charge, which encourages the deposition of aerosol particles on the inside walls of the spacer. However, after repeated use, the deposition of drug on the surface of the spacer has an anti-static effect. Thus, the dose available to the patient is likely to be strongly influenced by the condition of the spacer, which will vary with time. Re-use of the spacer will initially be associated with increasing drug availability, but the situation is complicated by regular cleaning of the spacer device, which is advised to the patients. This is likely to reduce the subsequent availability of drug from the spacer, by permitting the further deposition of aerosol particles on its newly cleaned walls. This could result in a cycle of greater drug availability, despite apparently consistent dosing. This is of major concern in children in whom drug delivery is already highly variable due to age – specific breathing patterns such as low tidal volume and inspiratory flow rates1. Also, any delay in inhaling the drug after actuation (as may happen in younger children with low tidal volumes) results in a faster fallout of aerosol in polycarbonate spacers, compared to non-static spacers. This, too, leads to a significant reduction in the dose available for inhalation.7
Increased Lung Deposition
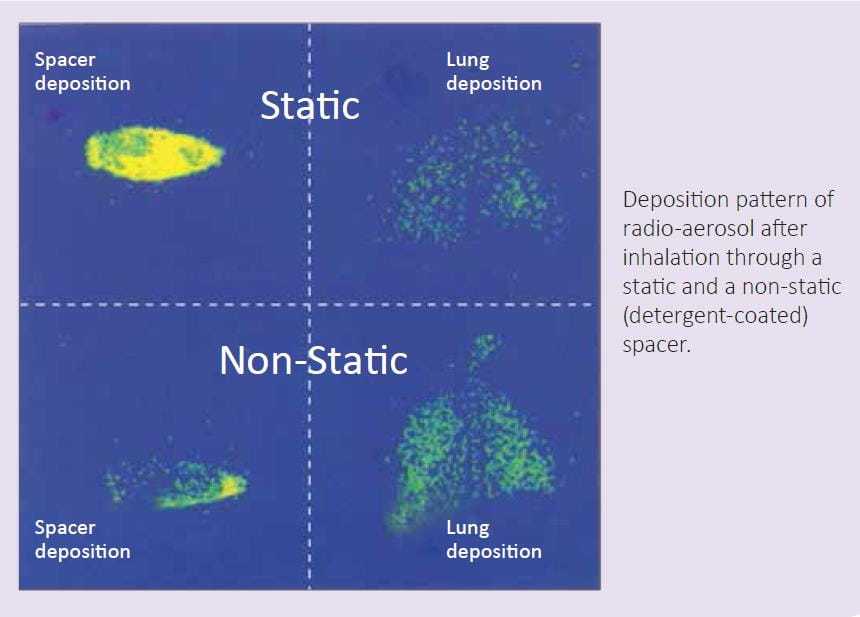
In an in vivo study, the influence of static charge on drug deposition in the lungs was evaluated using a detergent-coated (non-static) and a non-coated plastic spacer (static). Lung deposition of radiolabeled salbutamol was assessed in healthy adults, using imaging techniques.
There was a three-fold increase in lung deposition from non-static spacers compared to static spacers (45.6% vs. 11.5%). The mean amount of salbutamol remaining in the static spacers was 76.7% compared to 33.1% in the non-static spacers (p <0.001).8
The first Gamma Scintigraphy Study in India
The lung deposition of inhaled salbutamol was assessed using the Asthalin Inhaler (salbutamol) with the Zerostat Spacer, using gamma scintigraphy. The test drug (salbutamol) was radiolabeled by using the radionuclide Technetium 99m. Immediately after inhaling the radiolabeled aerosol, the subject had the imaging performed, using a gamma camera.
The mean percentage of salbutamol deposited in the lungs was found to be 11% with the pMDI alone; this was increased to almost 24% with the use of the Zerostat Spacer. Also, the Zerostat Spacer reduced the oropharyngeal deposition of the drug, as can be seen from the figure.9
Higher Deposition in Children
Another study evaluated the plasma salbutamol levels in children before and 5, 10, 15 and 20 minutes after inhalation from a static (350 ml and 145 ml) and a non-static (350 ml) spacer coated with benzalkonium chloride. The non-static spacer was found to deliver a significantly (p <0.05) higher lung dose in comparison to the other two spacers. Electrostatic charge in plastic spacers reduced the delivered lung dose in children by at least two fold.10
Greater Bronchodilation
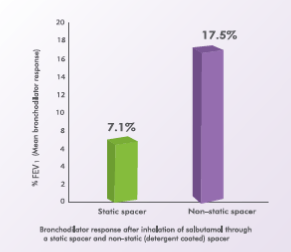
A randomized, double-blind, placebo-controlled, crossover trial evaluated the effect of a static charge on bronchodilator response to salbutamol. Accordingly, 12 asthmatic children, aged 13 to 17 years, inhaled 200 mcg of salbutamol from a pMDI with a static spacer and from a pMDI with a non-static (detergentcoated) spacer. The mean bronchodilator response after inhalation of salbutamol from the static spacer was 7.1% while that with the non-static spacer was 17.5%.11
Inhalation technique: Has Less Impact in Non-static Spacers
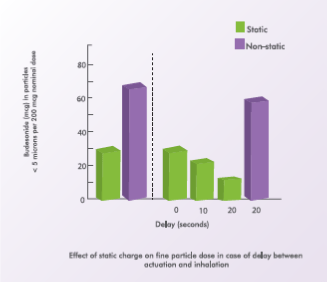
Compared to actuating a single dose into the spacer and then inhaling, firing multiple doses and then inhaling these doses in a single breath has been reported to reduce drug delivery. Also, longer the interval between actuation and inhalation, lesser is the drug delivered. This is especially seen with static spacers. A 20-second delay causes a two-thirds reduction in drug delivery.7
Non-static spacers can overcome the drawbacks inherent in static spacers. For example, the half-life for aerosol “fall-out” within the spacer chamber is greatly increased with non-static spacers.
The longer residence time of the actuated aerosol gives infants and children with impaired respiration (especially small tidal volumes and low inspiratory flows) a better opportunity to inhale the aerosol cloud. All the international guidelines have also acknowledged the role of anti-static spacers.
pMDI + Spacer: As Effective as a Nebulizer
Several clinical studies have shown that spacers are at least as effective as nebulizers in the treatment of severe acute asthma attacks. Results of several studies indicate that MDIs with spacers are as effective as nebulizers in the treatment of asthma. During an acute attack the use of high dose (10–15 puffs) short-acting beta2 agonist via an MDI and large volume spacer is an effective alternative to its use via a nebulizer. In addition, cost analysis studies indicate that for hospitalized adult patients with asthma exacerbations, treatment with either MDIs or nebulizers produce equivalent responses, and MDI use is not associated with longer periods of hospitalization.
A randomized, double-blind trial has reported pMDIs with spacers to be as effective as nebulizers for the delivery of bronchodilators for emergency department treatment of wheezing in children (n = 85) aged 2 to 24 months. Compared with the spacer group, the nebulizer group had a significantly higher mean initial pulmonary index score* (7.6 + 2.5 vs. 6.6 + 2.0; p = 0.002). After controlling for the baseline pulmonary index score, there were fewer hospital admissions in the spacer group (5% vs. 20%; p = 0.05).
According to the study investigators, “the introduction of pMDIs with spacers has provided a more efficient, cost-effective and easier way of delivering salbutamol to infants and young children.”12
*Pulmonary index score is a validated asthma severity score. In this study, mild asthma had a pulmonary index score of zero to 3, moderate asthma had a score of 4 to 7, and severe asthma had a score of 8 to 12.
Transparent Zerostat VT Spacer
The Zerostat VT is a novel holding chamber, which is valved, non-static and transparent.
It is small, portable, child-friendly, easy to carry and easy to clean. The Zerostat VT Spacer has all the advantages of a spacer, including overcoming coordination problems, reducing the spray effect at the back of the throat, reducing oro-pharyngeal deposition and enhancing lung deposition of the drug.
It is made of a non-static polyamide material, which helps in delivering a greater amount of medicine into the lungs.
As it is transparent, it enables the aerosol plume to be clearly seen following inhaler actuation.
It is diamond-shaped; thus, its shape matches the shape of the aerosol plume discharged from the pMDI.
It has a volume of 280 ml, which is ideal for adults and also for children having low tidal volumes.
It has a length of ~18 cm.
It contains a one-way, low-resistant valve. The valve functions at low inspiratory flow rates of 20 L/min. The one-way valve opens upon inhalation and closes during exhalation, thus preventing the dilution of the drug in the chamber and ensuring its retention in the spacer for subsequent inhalation.
The half life* (t½) of the Zerostat VT Spacer is approximately 60 seconds. Hence, it gives more lag time for inhalation as compared to static spacers.
*Half life (t½) of the spacer is the time lag between actuation and inhalation at which the percentage ½ of fine particle mass drops to half of its original value.
Steps to use Zerostat VT Spacer
To assemble your Zerostat VT spacer, firmly push the two halves of the spacer together and rotate with the mouthpiece cap in place. The Zerostat VT spacer comes with two locks which ensure proper assembling of the two halves.
Remove the mouthpiece cap from the mouthpiece of the inhaler. Shake the inhaler well.
Insert the inhaler firmly into the opposite end of the Zerostat VT spacer
Holding the inhaler, press down on the canister to release a dose into the Zerostat VT spacer. You will see the dose released in the spacer
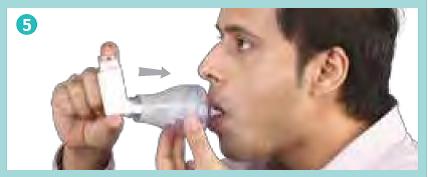
Remove the mouthpiece cap from the Zerostat VT spacer, breathe out fully and close your lips firmly around the mouthpiece to create a good seal as shown in the picture, (do not bite it) breathe in deeply through your mouth, thus inhaling the medicine through the spacer.
Remove the Zerostat VT spacer from your mouth and hold your breath for about 10 seconds, or as long as is comfortable. Breathe out slowly. If a second dose is required, wait for a minute, repeat steps 2 to 5
For Children
Children should use the Zerostat VT spacer under parental guidance
For children below 3 years a face mask (Baby Mask) attached to the Zerostat VT spacer should be used to facilitate administration
Features of the Zerostat VT Spacer
Notch
Two improved notches provided for fitting the two halves of the Zerostat VT spacer firmly
CTP*
Unique transparent static free material, which increases drug available for inhalation
*CTP – Customised Thermoplastic Polymer
Flow Gate Valve
Flow gate valve prevents the patient from exhaling into the spacer
pMDI Insertion Port
New improved design allows snug fit with pMDI
Mouthpiece
Designed to give a comfortable fit during inhalation
Attached Dust Cap
New hinged dust cap makes it easier to protect mouthpiece from contamination
Air Vents
Reduces resistance when patient exhales
Transparent Minizerostat Spacer
The Minizerostat spacer is a valved holding chamber with a volume of 150 ml.
It is made up of antistatic polyamide material which helps to deliver greater amount of drug into the lungs.
It is a single unit spacer, thus reducing the need for assembly which makes it easy to use and maintain.
The unidirectional silicone valve prevents back flow of air and helps deliver more drug.
The air vents help ensures that there is no dilution of the dose when the patient exhales into the spacer.
Pear shape of the spacer helps minimize impaction on the walls of the spacer. It is about 30% more compact and hence easy to carry
The half-life* (t½) of the Minizerostat Spacer is approximately 45 seconds
*Half life (t½) of the spacer is the time lag between actuation and inhalation at which the percentage ½ of fine particle mass drops to half of its original value.
Steps to Use the Minizerostat Spacer
Remove the mouthpiece cap from the mouthpiece of the inhaler. Shake the inhaler well.
Insert the inhaler firmly into the opposite end of the Minizerostat Spacer.
Breathe out fully. Remove the mouthpiece cap from the Minizerostat Spacer and place the mouthpiece in your mouth. Close your lips firmly around the mouthpiece to create a good seal as shown in the picture (Do not bite it).
Press down the canister of the inhaler to release a dose of medicine into the Minizerostat Spacer. You will see the dose released in the spacer.
Breathe in slowly and deeply through your mouth, thus inhaling the medicine through the Minizerostat Spacer.
For Children
Children should use the Minizerostat Spacer under adult guidance
For children below 4 years of age, a face mask (Baby Mask/Infant Mask) attached to the Minizerostat Spacer should be used to facilitate administration.
Features of the Minizerostat Spacer
Air Vents
8 air vents to ensure there is no blow back into the spacer
CTP*
Unique transparent static free material, which increases drug available for inhalation
*CTP – Customised Thermoplastic Polymer
Unidirectional Silicon Valve
Prevents black flow of air and helps to deliver more drug
Pear Shaped
Minimises impaction on the side walls
Single unit Spacer
For easy usage and maintenance
Dust Cap
Dust cap ensures protection of the mouthpiece from contamination
Mouthpiece
Designed to give a comfortable fit during inhalation
Universal pMDI Port
Universal pMDI port allows snug fit with pMDI
References
- Clin Pharmacokinet 2004; 43(6): 349-60
- Am J Health-Syst Pharm 2001; 58:585
- Global Initiative for Asthma (GINA) 2017
- Cochrane Review 2013; DOI 10.1002/14651858.CD000052.pub3
- Indian Pediatr. 1997 Jun;34(6):497-503.
- Jr Aerosol Med 2014; 27(1): S4-S23
- Br J Clin Pharmacol 1995; 40:76-78 8.
- Eur Respir J 1999; 13: 673-678
- Data on file, Cipla Ltd.
- Br. J Clin Pharmacol 1999; 47: 333-39 11.
- Swiss Med Wkly 2 0 0 1; 1 3 1: 1 4 – 1 8
- Pulm Pharmacol Ther 2001; 14:351-366