Prescriber's Guide
Majority of breast cancer-related deaths are a result of complications from recurrent or metastatic disease. Most
women with metastatic breast cancer (MBC) have a limited survival time of 18-24 months, and only 20% of them
would be ative 5 years after the initial diagnosis of distant metastases has been made.
The treatment of MBC includes chemotherapy, hormonal therapy, radiotherapy, bisphosphonates, biological therapy
or combinations of these, supportive care and, in certain cases, resection of primary tumor and/or metastases.
Since treatment of metastatic disease is not given with curative intent, the main goals of palliative care are
to prolong progression-free survival (PFS) and overall survival (OS), reduce disease symptoms and achieve the
best possible quality of life (QOL) for the patient.
Taxanes and anthracyclines remain the cornerstone of the treatment of early and locally advanced breast cancer.
Because the taxanes are highly hydrophobic, two classic anti-cancer agents, paclitaxel and docetaxel, are
formulated into aqueous injections with the help of solvents. Distinguished characteristic of the solvent-based
delivery system is the large amounts of the conventional surfactants and solvents contained within the
formulation, which may cause serious side effects, including hypersensitivity reactions, neutropenia and
neuropathy, even death. More recently, it has been recognized that these solvents may adversely affect efficacy
because of entrapment of active drug in micelles formed in the plasma compartment, leading to increased systemic
drug exposure, decreased drug clearance, nonlinear pharmacokinetics, and lack of dose-dependent antitumor
activity. Drug entrapment affects not only the taxanes but also the co administered drugs (e.g., anthracyclines)
and, thus, is an important consideration in the design of combination therapies. Exploiting an improved
formulation of anti-cancer agents was necessary to administer the drug more effectively and safety.
Paclitaxel nanoparticle albumin bound is polyethoxylated castor oil-free novel formulation of paclitaxel that
delivers the drug in the form of a nanoparticle colloidal suspension. It offers several improvements over the
conventional, solvent- and polyethoxylated castor oil- based paclitaxel, including lower toxicities, shorter
administrating time, higher efficacy and without premedication. Albumin in it serves not onfy as a targeting
ligand, but also as a carrier in drug delivery and a stabilizer in the formulation.
- Oncologist.20O5;10(3):20-9
- Cancer Treat Rev. 2010:360133-42
- Recent Pal Anbcaneer Drug Discav.20G9;4|3|:262-72
- JCin Cnco,2005;1;23<31);7794-e03
- Cnco Targets Ther. 2011;4:123-36
Paclitaxel is an antimicrotubule agent that promotes the assembly of microtubule: from tubulin dimers and
stabilises microtubules by preventing depolymerisatior This stability results in the inhibition of the normal
dynamic reorganisation of th< microtubule network that is essential for vital interphase and mitotic cellule
functions. In addition, paclitaxel induces abnormal arrays or "bundles" c microtubules throughout the
ceil cycle and multiple asters of microtubules during mitosis.
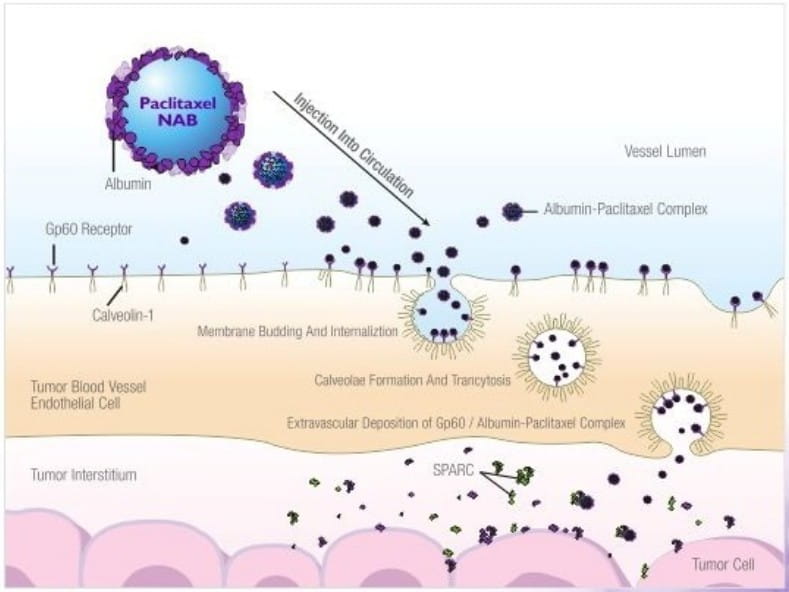
In this paclitaxel nanoparticle albumin bound formulation, the paclitaxel is present in a non-crystalline,
amorphous state. Albumin plays an important role in endothelial transcylosis of protein bound and unbound plasma
constituents mainly by binding to a cell-surface 60 kDa glycoprotein receptor (gp60) on the endothelial cell
membrane. This leads to activation of caveolin-1. a major component of membrane vesicles, resulting in receptor
mediated internalization of the albumin-drug complex into caveolae (small invaginations of plasma membrane).
Subsequently, caveolae transports the albumin-drug conjugate to the extracellular space, including the tumor
interstitium. SPARC {secreted protein, acidic and rich in cysteine), which is believed to be selectively
secreted by the tumors, binds to albumin-drug complex with the resultant release of the drug in the vicinity of
tumor cells.
Albumin enhances transport of paclitaxel (antimicrotubule agent) across endothelial cells and SPARC helps
accumulation of paclitaxel in the area of tumour.
The pharmacokinetics of total paclitaxel following 30- and 180-minute infusions of paclitaxel nanoparticle
albumin bound at dose levels of 80 to 375 mg/m2 were determined in clinical studies. The paclitaxel
exposure (AUC) increased linearly from 2653 to 16736 ng.hr/ml following dosing from 80 to 300 mg/m2.
Following intravenous administration of paclitaxel nanoparticle albumin bound to patients with metastatic breast
cancer at the recommended clinical dose of 260 mg/m2, paclitaxel plasma concentrations declined in a
multiphasic manner. The mean Cmax. of paclitaxel, which occurred at the end of the infusion, was 18.7
pg/ml. The mean total clearance was 15 l/hr/m2. The terminal half-life was about 27 hours. The mean
volume of distribution was 632 l/m2; the large volume of distribution indicates extensive extra
vascular distribution and/or tissue binding of paclitaxel.
In a study in patients with advanced solid tumours, the pharmacokinetic characteristics of paclitaxel following
paclitaxel nanoparticle albumin bound administered intravenously at 260 mg/m2 over 30 minutes were
compared with those following 175 mg/m2 of the sotvent-based paciitaxel injection administered over 3
hours. The clearance of paciitaxel with paciitaxel nanoparticie albumin bound was larger (43%) than that
following a solvent-based paciitaxel injection and its volume of distribution was also higher (53%). Differences
in Cmax and Cmax corrected for dose reflected differences in total dose and rate of
infusion. There were no differences in terminal half-lives.
In a repeat dose study with 12 patients receiving paclitaxel nanoparticle albumin bound administered
intravenously at the approved dose, intrapatient variability in systemic paclitaxel exposure (AUCint)
was 19% (range = 3.21 %-27.70%). There was no evidence for accumulation of paclitaxel with multiple treatment
courses.
An analysis of patient exposure (AUCint) against bodyweight indicated a trend toward reduced AUC at
260 mg/m2 paclitaxel nanoparticle albumin bound, with decreased body weight. Patients weighing 50 kg
had paclitaxel AUC approximately 25% lower than those weighing 75 kg. The clinical relevance of this finding is
uncertain.
The protein binding of paclitaxel following paclitaxel nanoparticle albumin bound was evaluated by ultra
filtration. The fraction of free paclitaxel was significantly higher with paclitaxel nanoparticle albumin bound
(6.2%) than with solvent-based paclitaxel (2.3%). This resulted in significantly higher exposure to unbound
paclitaxel with paclitaxel nanoparticle albumin bound compared with solvent-based paclitaxel, even though the
total exposure is comparable. This is possibly due to paclitaxel not being trapped in polyethoxylated castor oil
micelles as with solvent-based paclitaxel. Based on the published literature, in vitro studies of binding to
human serum proteins, (using paclitaxel at 6uM) the presence of ranitidine, dexamethasone, or diphenhydramine
did not affect protein binding of paclitaxel.
Based on the published literature, in vitro studies with human liver microsomes and tissue slices show that
pactitaxel is metabolised primarily to 6a-hydroxypaclitaxel; and to two minor metabolites.
3'-p-hydroxypaclitaxe! and 6a-3'-p-dihydroxypaclitaxel. The formation of these hydroxylated metabolites
is catalysed by CYP2C8, -3A4. and both -2C8 and -3A4 respectively.
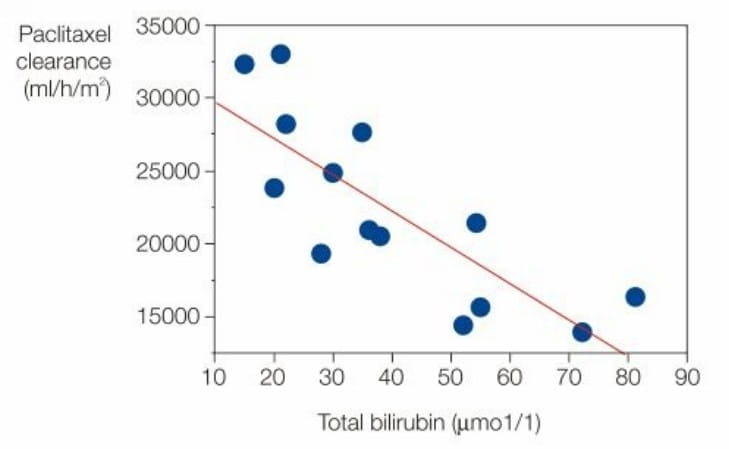
The pharmacokinetic profile of paclitaxel nanoparticle albumin bound administered as a 30 minute infusion was
evaluated in 15 out of 30 patients with three levels of hepatic impairment based on serum bilirubin and liver
enzyme levels. (3,5,6,7)
Figure below shows the correlation between paclitaxel clearance and total blood bilirubin as measured just prior
to dosing.
The effect of renal dysfunction on the disposition of paclitaxel has not been formally investigated.
In patients with metastatic breast cancer, after a 30 minute infusion of paclitaxel nanoparticle albumin bound at
260 mg/m2 the mean value for cumulative urinary excretion of unchanged active substance accounted for
4% of the total administered dose with less than 1% as the metabolites 6a-hydroxypaclitaxel and
3'-p-hydroxypaclitaxel, indicating extensive non-renal clearance. Paclitaxel is principally eliminated by
hepatic metabolism and biliary excretion.
Pharmacokinetics of paclitaxel in patients aged over 65 years seems comparable to that in patients less than 65
years. However, little information in patients over 75 years is available as only 3 patients over 75 years of
age where included in the pharmacokinetic analysis.
Clearance of paclitaxel with paclitaxel nanoparticle albumin bound was larger and its volume of distribution was
higher than that following a solvent-based paclitaxel injection.
Fraction of free paclitaxel was significantly higher with paclitaxel nanoparticle albumin bound (6.2%) than with
solvent-based paclitaxel (2.3%) resulting in significantly higher exposure to unbound paclitaxel.
Pharmacokinetics of paclitaxel in patients aged over 65 years seems comparable to that in patients less than 65
years.
- Recent Pal Anbcaneer Drug Discav.20G9;4|3|:262-72
- EMC www.medicines.org. uk/
- DailyMed dailymed.nfcn.nih.gov/
- Breast Cancer (Auckl). 2011 ;5:63-66
- Adapted from http://primeancology.org/Files/Doc/SLIDES/EBCC2010/Awada-nab-technology_platform.pdf
Paclitax NAB is indicated for the treatment of breast cancer after failure of combination chemotherapy
for metastatic disease or relapse within 6 months of adjuvant chemotherapy. Prior therapy should have
included an anthracycline unless clinically contraindicated.
The recommended dose of Paclitax NAB is 260 mg/m2 administered intravenously over 30 minutes
every 3 weeks.
Dose Adjustments During Treatment
- Patients who experience severe neutropenia (neutrophil count < 500 cells/mm3for a week or
longer) or severe sensory neuropathy during Paclitax NAB therapy should have the dose reduced to 220
mg/m2 for subsequent courses.
- Following recurrence of severe neutropenia or severe sensory neuropathy, additional dose reduction should be
made to 180 mg/m2
- Paclitax NAB should not be administered until neutrophil counts recover to > 1,500
cells/mm3.
- For grade 3 sensory neuropathy withhold treatment until resolution to grade 1 or 2, followed by a dose
reduction for ail subsequent courses.
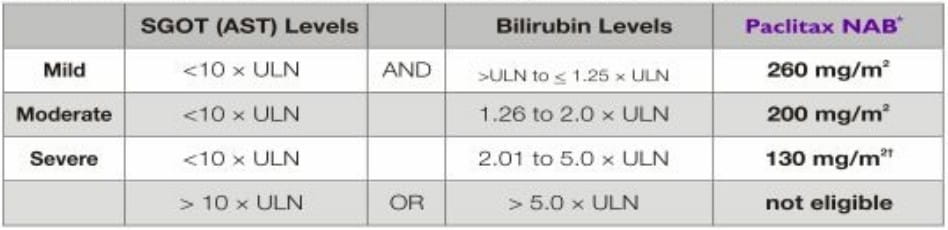
Patients with Hepatic Impairment
- No dose adjustment is necessary for patients with mild hepatic impairment.
- Patients with moderate and severe hepatic impairment treated with Paclitax NAB may be at increased
risk of toxicities known to paclitaxel.
- Patients should not receive Paclitax NAB if AST > 10 x ULN or bilirubin > 5.0 x ULN.
- The dose of Paclitax NAB can be increased up to 200 mg/m2 in patients with severe hepatic
impairment in subsequent cycles based on individual tolerance.
- Patients should be monitored closely.
Patients with Impaired Renal Function
Studies in patients with impaired renal function have not been performed and insufficient data are currently
available to recommend dose modifications in patients with renal impairment.
The recommended dose of Paclitax NAB is 260 mg/m2 I.V. over 30 minutes every 3 weeks in the treatment
of breast cancer after failure of combination chemotherapy for metastatic disease or relapse within 6 months of
adjuvant chemotherapy.
Dose modifications for patients with severe & recurrent neuropathy, neutropenia or moderate & severe
hepatic impairment should be done.
Paclitax NAB should only be administered under the supervision of a qualified oncologist in units specialised in the
administration of cytotoxic agents.
Preparation and Administration Precautions
Paclitax NAB is a cytotoxic anticancer drug and, as with other potentially toxic paclitaxel compounds,
caution should be exercised in handling Paclitax NAB. The use of gloves is recommended, if Paclitax
NAB (lyophilized cake or reconstituted suspension) contacts the skin, wash the skin immediately and
thoroughly with soap and water. Following topical exposure to paclitaxel, events may include tingling, burning
and redness. If Paclitax NAB contacts mucous membranes; the membranes should be flushed thoroughly with
water.
Given the possibility of extravasation, it is advisable to closely monitor the infusion site for possible
infiltration during drug administration. Limiting the infusion of Paclitax NAB to 30 minutes, as
directed, reduces the likelihood of infusion-related reactions.
No premedication to prevent hypersensitivity reactions is required prior to administration of PaclitaxNAB.
Use of gloves while handling Paclitax NAB is recommended.
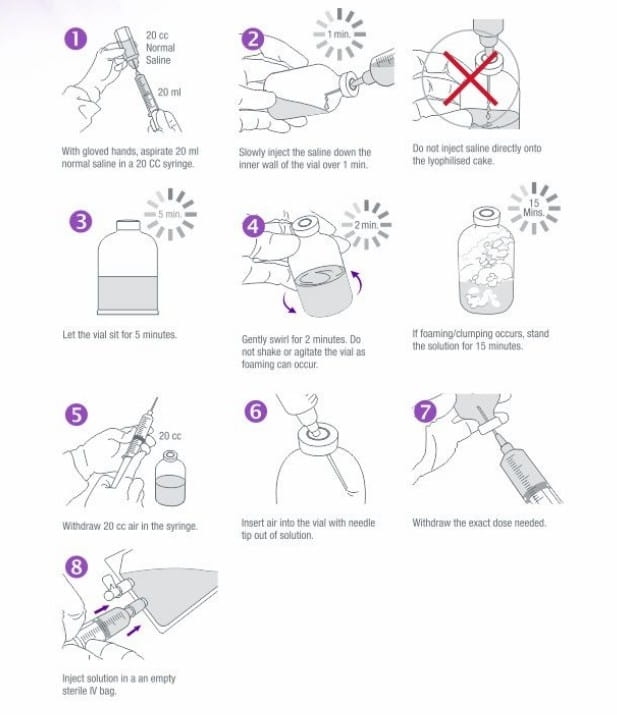
Preparation for Intravenous Administration
EMC www.medicines.org. uk/
DailyMed dailymed.nfcn.nih.gov/
Breast Cancer (Auckl). 2011 ;5:63-66
- Paclitaxel nanoparticle albumin bound should not be used in patients who have baseline neutrophil counts of
< 1,500 cells/mm3.
- Hypersensitivity to the active substance or to any of the excipients.
- Lactation.
Cnco Targets Ther. 2011;4:123-36
EMC www.medicines.org. uk/
DailyMed dailymed.nfcn.nih.gov/
Paclitaxel nanoparticle albumin bound may have substantially different pharmacological properties compared to
other formulations of paclitaxel. It should not be substituted for or with other paclitaxel formulations.
(6,7)
Hypersensitivity
If hypersensitivity occurs, the medicinal product should be discontinued immediately, symptomatic treatment
should be initiated, and that patient should not be rechallenged with paclitaxel.
Haematology
Bone marrow suppression (primarily neutropenia) occurs frequently with paclitaxel nanoparticle albumin bound.
Neutropenia is dose-dependent and a dose-limiting toxicity. Frequent monitoring of blood cell counts should be
performed during paclitaxel nanoparticle albumin bound therapy. Patients should not be retreated with subsequent
cycles of paclitaxel nanoparticle albumin bound until neutrophils recover to > 1,500 cells/mm3 and
platelets recover to > 100,000 cells/mm3.
Neuropathy
Sensory neuropathy occurs frequently with paclitaxel nanoparticle albumin bound, although development of severe
symptoms is less common. The occurrence of grade 1 or 2 sensory neuropathy does not generally require dose
reduction. If grade 3 sensory neuropathy develops, treatment should be withheld until resolution to grade t or 2
followed by a dose reduction for all subsequent courses of paclitaxel nanoparticle albumin bound is recommended.
Cardiotoxicity
Rare reports of congestive heart failure and left ventricular dysfunction have been observed among individuals
receiving paclitaxel nanoparticle albumin bound. Most of the individuals were previously exposed to cardiotoxic
drugs, such as anthracyclines, or had underlying cardiac history. Thus patients receiving paclitaxel
nanoparticle albumin bound should be vigilantly monitored by physicians for the occurrence of cardiac events.
CNS Metastases
The effectiveness and safety of paclitaxel nanoparticle albumin bound in patients with central nervous system
(CNS) metastases has not been established. CNS metastases are generally not well controlled by systemic
chemotherapy.
Gastrointestinal Symptoms
If patients experience nausea, vomiting and diarrhoea following the administration of paclitaxel nanoparticle
albumin bound, they may be treated with commonly used anti-emetics and constipating agents.
Excipients
When reconstituted, paclitaxel nanoparticle albumin bound contains approximately 425 mg sodium per dose. To be
taken into consideration by patients on a controlled sodium diet.
Drug Interactions
No drug interaction studies have been conducted with paclitaxel nanoparticle albumin bound. The metabolism of
paclitaxel is catalyzed by CYP2C8 and CYP3A4. In the absence of formal clinical drug interaction studies,
caution should be exercised when administering paclitaxel nanoparticle albumin bound concomitantly with
medicines known to inhibit (e.g. ketoconazole and other imidazole antifungals, erythromycin, fluoxetine,
gemfibrozil, cimetidine, ritonavir, saquinavir, indinavir, and netfmavir) or induce (e.g. rifampicin,
carbamazepine, phenytoin, efavirenz, nevirapine) either CYP2C8orCYP3A4.
Renal Impairment
The effect of renal dysfunction on the disposition of paclitaxel nanoparticle albumin bound has not been
investigated.
Hepatic Impairment
Because the exposure and toxicity of paclitaxel can be increased with hepatic impairment, administration of
paclitaxel nanoparticle albumin bound in patients with hepatic impairment should be performed with caution. The
starting dose should be reduced for patients with moderate and severe hepatic impairment as directed in dosage
and administration.
Pregnancy
Category D
There are very limited data on the use of paclitaxel in human pregnancy. Paclitaxel is suspected to cause
serious birth defects when administered during pregnancy. Studies in animals have shown reproductive toxicity.
Paclitaxel nanoparticle albumin bound should not be used in pregnancy, and in women of childbearing potential
not using effective contraception, unless the clinical condition of the mother requires treatment with
paclitaxel.
Lactation
It is not known if paclitaxel is excreted in human milk. Because of potential serious adverse reactions in
breast-feeding infants, paclitaxel nanoparticle albumin bound is contraindicated during lactation. Breastfeeding
must be discontinued for the duration of therapy.
Paediatric Use
The safety and efficacy of paclitaxel nanoparticle albumin bound in children has not been established. There is
no relevant use of paclitaxel nanoparticle albumin bound in the paediatric population in the indication of
metastatic breast cancer.
Geriatric Use
In the clinical studies, no toxicities occurred notably more frequently among elderly patients who received
paclitaxel nanoparticle albumin bound.
Use in Males
Men should be advised to not father a child while receiving treatment with paclitaxel nanoparticle albumin
bound.
Albumin (Human)
Paclitaxel nanoparticle albumin bound contains albumin (human), a derivative of human blood. Based on effective
donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of
viral diseases. A theoretical risk for transmission of Creutzfeldt - Jakob disease (CJD) also is considered
extremely remote. No cases of transmission of viral diseases or CJD have ever been identified for albumin.
Injection Site Reaction
Injection site reactions occur infrequently with paclitaxel nanoparticle albumin bound and were mild in the
randomized clinical trial. Given the possibility of extravasation, it is advisable to closely monitor the
infusion site for possible infiltration during drug administration.
Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of paclitaxel nanoparticle albumin bound has not been studied.
Paclitaxel has been shown to be clastogenic in vitro (chromosome aberrations in human lymphocytes) and in vivo
(micronucleus test in mice). Paclitaxel nanoparticle albumin bound was not mutagenic in the Ames test or the
CHO/HGPRT gene mutation assay.
Paclitaxel nanoparticle albumin bound induced infertility in male rats. Male patients should seek advice on
conservation of sperm prior to treatment because of the possibility of irreversible infertility due to therapy
with paclitaxel nanoparticle albumin bound.
Sexually active men and women should use effective methods of contraception during treatment and up to six months
after treatment for men, and one month after treatment for women.
Ability to Drive and Use Machines
Adverse events such as fatigue, lethargy, and malaise may affect the ability to drive and use machines.
Paclitaxel nano particle albumin bound should be discontinued:
- When hypersensitivity occurs,
- In case of neutropenia, until neutrophil counts recover to >1,500 cells/mm3 and platelets
recover to > 100,000 cells/mm3.
- When grade 3 sensory neuropathy occurs until resolution to grade 1 or 2 followed by a dose reduction for all
subsequent courses.
Patients with nausea, vomiting and diarrhoea may be treated with anti-emetics & constipating
agents.
Cardiac events and blood cell counts must be vigilantly monitored.
Pregnancy category D and contraindicated during lactation.
The following are the most common and important incidences of adverse reactions related to 229 patients with
metastatic breast cancer who were treated with 260 mg/m2paclitaxel nanoparticle albumin bound once
every three weeks in the pivotal phase III clinical study. (4,5,6,7,8)
Blood and lymphatic system disorders
Neutropenia was the most notable important haematological toxicity (reported in 79% of patients), and was
rapidly reversible and dose dependent; leucopenia was reported in 71 % of patients. Grade 4 neutropenia (<500
cells/mm3) occurred in 9% of patients treated with paclitaxel nanoparticle albumin bound. Febrile
neutropenia occurred in four patients on paclitaxel nanoparticle albumin bound. Anaemia (Hb < 10 g/dl) was
observed in 46% of patients on paclitaxel nanoparticle albumin bound, and was severe (Hb < 8 g/dl) in three
cases. Lymphopenia was observed in 45% of the patients.
Nervous system disorders
In general, the frequency and severity of neurotoxicity was dose-dependent in patients receiving paclitaxel
nanoparticle albumin bound. Peripheral neuropathy (mostly Grade 1 or 2 sensory neuropathy) was observed in 68%
of patients on paclitaxel nanoparticle albumin bound with 10% being Grade 3, and no cases of Grade 4.
Gastrointestinal disorders
Nausea occurred in 29% of the patients and diarrhoea in 25% of the patients.
Skin and subcutaneous tissue disorders
Alopecia was observed in 90% of the patients treated with paclitaxel nanoparticle albumin bound.
Musculoskeletal and connective tissue disorders
Arthralgia occurred in 32% of patients on paclitaxel nanoparticle albumin bound and was severe in 6% of cases.
Myalgia occurred in 24% of patients on paclitaxel nanoparticle albumin bound and was severe in 7% of cases. The
symptoms were usually transient, typically occurred three days after paclitaxel nanoparticle albumin bound
administration and resolved within a week.
General disorders and administration site conditions
Asthenia/Fatigue was reported in 40% of the patients. Table shows the list of adverse reactions associated with
the administration of paclitaxel nanoparticle albumin bound to patients from studies in which paclitaxel
nanoparticle albumin bound has been administered as a single agent at any dose in any indication (N = 789).
The frequency of undesirable effects listed in table is defined using the following convention:
Very common (>1/10); common (>1/100 to <1/10); uncommon ( >1/1,000 to <1/100); rare (>1/10,000
to < 1/1,000); very rare (< 1/10,000), not known (cannot be estimated from the available data).
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Infections and Infestations
Common: Infection, urinary tract infection, folliculitis, upper respiratory tract infection, candidiasis,
sinusitis Uncommon: Oral candidiasis, nasopharyngitis, cellulitis, herpes simplex, viral infection, pneumonia,
catheter-related infection, fungal infection, herpes zoster, injection site infection
Neoplasm Benign, Malignant and Unspecified (Including Cysts and Polyps)
Uncommon: Metastatic pain, tumour necrosis
Blood and lymphatic System Disorders
Very Common: Neutropenia, anaemia, leucopoenia. thrombocytopenia, lymphopenia, bone marrow suppression
Common: Febrile neutropenia
Rare: Pancytopenia
Immune System Disorders
Uncommon: Hypersensitivity Rare: Severe hypersensitivity
Metabolism and Nutrition Disorders
Very common: Anorexia
Common: Dehydration, decreased appetite, hypokalemia Uncommon: Hypophosphataemia, fluid retention, hypoalbuminaemia,
polydipsia, hyperglycaemia, hypocalcaemia, hypoglycemia, hyponatremia
Psychiatric Disorders
Common: Insomnia, depression, anxiety Uncommon: Restlessness
Nervous System Disorders
Very Common: Peripheral neuropathy, neuropathy. hypoaesthesia. paresthesia.
Common: Peripheral sensory neuropathy, headache, dysgeusia. dizziness, peripheral motor neuropathy, ataxia.
sensory disturbance, somnolence.
Uncommon: Polyneuropathy, areflexia, dyskinesia. hyporeflexia, neuralgia, sensory loss, syncope, postural
dizziness, neuropathic pain, tremor
Eye Disorders
Common: Increased lacrimation. blurred vision, dry eye. keratoconjunctivitis sicca, madarosis
Uncommon: Eye irritation, eye pain, abnormal vision, reduced visual acuity, conjunctivitis, visual disturbance,
eye pruritus, keratitis
Ear and Labyrinth Disorders
Common: Vertigo
Uncommon: Ear pain, tinnitusCardiac disordersCommon: Tachycardia, arrhythmia, supraventricular tachycardia
Rare: bradycardia, cardiac arrest, left ventncular dysfunction. congestive heart failure
Vascular Disorders
Common: Flushing, hot flushes, hypertension, lymphoedema Uncommon: Hypotension, peripheral coldness, orthostatic
hypotension
Rare: Thrombosis
Respiratory, Thoracic and Mediastinal Disorders
Common: Dyspnoea, epistaxis. pharyngolaryngeal pain, cough, rhinitis, rhinorrhoea
Uncommon: Productive cough, exertional dyspnoea, sinus congestion, decreased breath sounds, pleural effusion,
allergic rhinitis, hoarseness, nasal congestion, nasal dryness, wheezing, pulmonary emboli, pulmonary
thromboembolism
Rare: Interstitial pneumonitis
Gastrointestinal Disorders
Very Common: Nausea, diarrhoea, vomiting, constipation, stomatitis
Common: Abdominal pain, abdominal distension, upper abdominal pain, dyspepsia, gastroesophageal reflux disease,
oralhypoaesthesia ncommon: Dysphagia, flatulence, glossodynia, dry mouth, gingival pain, loose stools,
oesophagitis, lower abdominal pain, mouth ulceration, oral pain, rectal haemorrhage
Hepatobiliary Disorders
Skin and Subcutaneous Tissue Disorders
Very Common: Alopecia, rash
Common: Nail disorder, pruritus, dry skin, erythema, nail pigmentation/discolouration, skin hyperpigmentation,
nycholysis, nail changes
Uncommon: Nail bed tenderness, urticaria, skin pain,photosensitivity reaction, pigmentation disorder, pruritic
rash, skin disorder, hyperhidrosis, onychomadesis, erythematousrash, generalised rash, dermatitis, night sweats,
macuto-papular rash, vitiligo, hypotrichosis, nail discomfort, generalized pruritus, macular rash, papular rash,
skin lesion,swollen face
Very rare: Stevens-Johnson syndromeb. toxic epidermal necrolysisb
Musculoskeletal and Connective Tissue Disorders
Very Common: Arthralgia, myalgia.
Common: Pain in extremity, bone pain, back pain, muscle cramps, limb pain
Uncommon: Chest wall pain, muscular weakness, neck pain,groin pain, muscle spasms, musculoskeletal pain, flank
pain, limb discomfort, muscle weakness
Renal and Urinary Disorders
Uncommon: Dysuna, pollakiuna, haematuria, nocturia, polyuria, urinary incontinence
Reproductive System and Breast Disorders
General Disorders and Administration Site Conditions
Very Common: Fatigue, asthenia, pyrexia.
Common: Peripheral oedema, mucosal inflammation, pain, rigors, oedema, weakness, decreased performance status,
chest parn. influenza-like illness, malaise, lethargy, hyperpyrexia
Uncommon: Chest discomfort, abnormal gait, swelling, injection site reaction
Investigations
Common; Decreased weight, increased alanine aminotransferase, increased aspartate aminotransferase, decreased
haematocrit. decreased red blood cell count, increased body temperature, increased gamma-glutamyttransferase,
increased blood alkaline phosphatase
Uncommon: Increased blood pressure, increased weight, increased blood lactate dehydrogenase, increased blood
creatinine, increased Wood glucose, increased blood phosphorus, decreased blood potassium, increased bilirubin
Injury, Poisoning and Procedural Complications
Uncommon: Contusion
Rare: Radiation recall phenomenon, radiation pneumonitisa
a-The frequency ofhypersesitivity reaction is calculated based on one definitely related case in a
population of 789 patients.
b- As reported in the post marketing surveilliance of paclitaxel nanoparticle albumin bound.
Unless otherwise noted, the following discussion refers to the adverse reactions that have been identified during
post-approval use of paclitaxel nanoparticle albumin bound. Because these reactions are reported voluntarily
from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish
a causal relationship to drug exposure. In some instances, severe events observed with paclitaxel injection may
be expected to occur with paclitaxel nanoparticle albumin bound.
Hypersensitivity Reactions
Severe hypersensitivity reactions have been reported with paclitaxel nanoparticle albumin bound. The use of
paclitaxel nanoparticle albumin bound in patients previously exhibiting hypersensitivity to paclitaxel injection
or human albumin has not been studied.
Cardiovascular
There have been reports of congestive heart failure and left ventricular dysfunction with paclitaxel
nanoparticle albumin bound. Most of the individuals were previously exposed to cardiotoxic drugs, such as
anthracyclines. or had underlying cardiac history.
Respiratory
There have been reports of interstitial pneumonia and pulmonary embolism in patients receiving paclitaxel
nanoparticle albumin bound and reports of radiation pneumonitis in patients receiving concurrent radiotherapy.
Reports of lung fibrosis have been received as part of the continuing surveillance of paclitaxel injection
safety and may also be observed with paclitaxel nanoparticle albumin bound.
Neurologic
Cranial nerve palsies and vocal cord paresis have been reported, as has autonomic neuropathy resulting in
paralytic ileus.
Vision Disorders
Reports in the literature of abnormal visual evoked potentials in patients treated with paclitaxel injection
suggest persistent optic nerve damage. These may also be observed with paclitaxel nanoparticle albumin bound.
Hepatic
Reports of hepatic necrosis and hepatic encephalopathy leading to death have been received as part of the
continuing surveillance of paclitaxel injection safety and may occur following paclitaxel nanoparticle albumin
bound treatment.
Gastrointestinal (Gl)
There have been reports of intestinal obstruction, intestinal perforation, pancreatitis, and ischemic colitis
following paclitaxel nanoparticle albumin bound treatment. There have been reports of neutropenic enterocolitis
(typhlitis), despite the coadministration of G-CSF, occurring in patients treated with paclitaxel injection
alone and in combination with other chemotherapeutic agents.
Injection Site Reaction
There have been reports of extravasation of paclitaxel nanoparticle albumin bound. Given the possibility of
extravasation, it is advisable to monitor closely the paclitaxel nanoparticle albumin bound infusion site for
possible infiltration during drug administration.
Severe events such as phlebitis, cellulitis, induration, necrosis, and fibrosis have been reported as part of the
continuing surveillance of paclitaxel injection safety. In some cases the onset of the injection site reaction
in paclitaxel injection patients either occurred during a prolonged infusion or was delayed by a week to ten
days. Recurrence of skin reactions at a site of previous extravasation following administration of paclitaxel
injection at a different site, i.e., "recall", has been reported.
There have been reports of conjunctivitis, cellulitis, and increased lacrimation with paclitaxel injection.
MOST COMMON TOXICITIES: Neutropenia, anaemia, leucopoenia, thrombocytopenia, lymphopenia, bone marrow
suppression, anorexia, peripheral neuropathy, neuropathy, hypoaesthesia, paraesthesia, nausea, diarrhoea,
vomiting, constipation, stomatitis, alopecia, rash, arthralgia, myalgia, fatigue, asthenia, pyrexia.
- JCin Cnco,2005;1;23<31);7794-e03
- Cnco Targets Ther. 2011;4:123-36
- EMC www.medicines.org. uk/
- DailyMed dailymed.nfcn.nih.gov/
- Breast Cancer (Auckl). 2011 ;5:63-66
There is no known antidote for paclitaxel overdose. In the event of an overdose, the patient should be closely
monitored. Treatment should be directed at the major anticipated toxicities, which are bone marrow suppression,
mucositis and peripheral neuropathy.
- EMC www.medicines.org. uk/
- DailyMed dailymed.nfcn.nih.gov/
Unopened vials of Paclitax NAB are stable until the date indicated on the package when stored between
20ºC to 25ºC (68ºF to 77ºF) in the original package. Neither freezing nor refrigeration
adversely affects the stability of the product.
Stability of Reconstituted Suspension in the Vial
Reconstituted Paclitax NAB in the vial should be used immediately, but may be refrigerated at 2ºC to
8ºC (36ºF to 46ºF) for a maximum of 8 hours if necessary. If not used immediately, each vial of
reconstituted suspension should be replaced in the original carton to protect it from bright light. Discard any
unused portion.
Stability of Reconstituted Suspension in the Infusion Bag
The suspension for infusion when prepared as recommended in an infusion bag should be used immediately but may
be stored at ambient temperature (approximately 25ºC) and lighting conditions for up to 4 hours. Discard
any unused portion.
- EMC www.medicines.org. uk/
- DailyMed dailymed.nfcn.nih.gov/
Store vial in its original carton below 25ºC. Store reconstituted suspension in its original carton at 2aC
to 8ºC to protect from light.
- EMC www.medicines.org. uk/
- DailyMed dailymed.nfcn.nih.gov/














