Principles of Inhalation Therapy
Introduction
The modern era of aerosol therapy began with the introduction of Medihaler Epi in 1956. Since then, aerosol therapy has revolutionized the treatment of several respiratory diseases, including obstructive airway diseases.
The advantages of inhalation therapy are direct delivery of drugs to the lungs, use of smaller doses, rapid onset of action and minimal systemic adverse effects.
A variety of inhalation devices are available in the market. To get the best results from these devices, it is very important that they are used correctly, for which it is essential to understand the principles of inhalation therapy and the various factors governing them.
Factors Affecting Pulmonary Drug Delivery
Device - Related Factors
- Particle size of the formulation
- Nature of the device (ease of use)
Patient - Related Factors
- Technique of use of the device
- Pattern of breathing
- Geometry of the airways
- Severity of disease
Importance of Particle Size in Aerosol Deposition particle size in aerosol deposit
An aerosol consists of a dispersion or suspension of solid particles or liquid droplets in a gaseous medium. Aerosols are now being used to treat lung, nasal and systemic diseases. Aerosol devices must generate drug particles of an appropriate size, such that they penetrate beyond the oropharynx and larynx and deposit in the lungs. Aerodynamic diameter is generally thought to be the most important particle-related factor that affects aerosol deposition.
Aerodynamic diameter is commonly applied to inhaled drug particles to predict where in the respiratorytract such particles will deposit. Upon entering the oral cavity, particles will deposit by impaction,sedimentation and Brownian motion depending on their size.
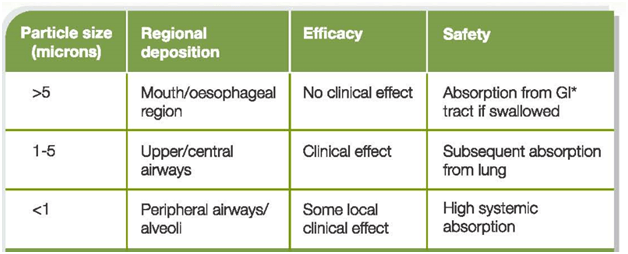
Table 1: Aerosol particle sizes with their deposition ,efficacy and safety
*Gastro-Intestinal
- Particles >5 μm are most likely to deposit by impaction in the oropharynx and be swallowed.
- Particles that are <5 μm have the greatest potential for deposition in the lungs. These particles of 1 – 5 μm size usually deposit by sedimentation or gravity. The proportion of particles within an aerosol that are <5 μm is often referred to as the fine-particle fraction (FPF), or the fine particle dose (FPD) if expressed in absolute mass of drug in particles <5 μm.
- Most particles of 0.1–1 μm diffuse by Brownian motion and deposit when they collide with the airway wall. The longer the residence time in the smaller, peripheral airways, the greater the deposition from sedimentation and Brownian motion processes.
Inhaled particles that do not deposit are exhaled.
Fate (Pharmacokinetics) of the Inhaled Drug
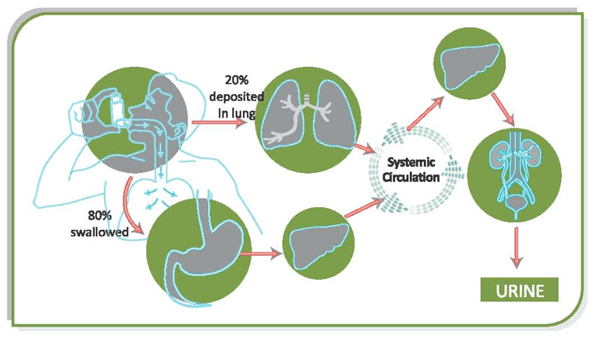
When the drug is administered by the inhalation route, approximately 15-20% of drug deposits in the lungs while approximately 80% of the drug deposits in the oropharynx, which then gets absorbed in the GI tract. Thus it can be seen that with a good technique, only about 20% of drug deposits in the lungs. So in case of poor inhaler technique, the lung deposition will be still less.
Figure:1 Pharmacokinetics of inhaled drug
The drug that enters the GI tract undergoes first pass metabolism in the liver. It can be seen that the drug reaching the systemic circulation is the drug coming from the lungs and from the GI tract. For optimum efficacy and safety of inhaled drugs, high pulmonary bioavailability coupled with low oral bioavailability is desirable. Steroids like fluticasone and ciclesonide and mometasone are almost completely metabolized in the liver and their oral bioavailability is less than 1%. This reduces the potential for any systemic side-effects.The drug is then metabolized and, finally, is eliminated out of the body.
Evaluation of Drug Deposition in the Lungs
In-vitro analysis is carried out to ascertain the quality of the manufactured product, and the analyses are usually conducted under strictly standardized conditions. Although the analyses are done in-vitro, it is often implied that the in-vitro results reflect the in-vivo situation. In-vitro testing allows many different variables within and between inhaler systems to be assessed rapidly and comparatively cheaply, without subjecting patients to the inconvenience and hazards of in-vivo testing.
Figure 2: Apparatus for in-vitro analysis
The most commonly used techniques include the Anderson cascade impactor, multi-stage liquid impinge and next-generation impactor (Figure 2).
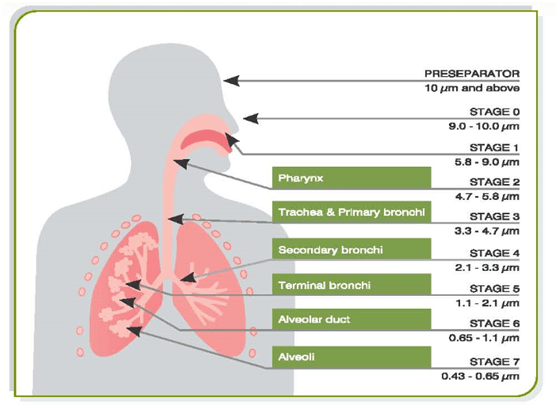
Figure 3: Anderson cascade impactor which stimulates human lungs
The cascade impactor consists of one or more stages normally arranged in the form of a stack in order of decreasing particle size. These separate the particles entrained in the aerosol stream passing through them into a series of size bands, broadly corresponding to their likely deposition sites in the respiratory tract.
The cascade impactor operates on the principle of inertial impaction. Each stage of the impactor comprises a single or series of nozzles or jets through which the sample-laden air is drawn, directing any airborne particle towards the surface of the collection plate for the particular stage.
At the end of the test, the particle mass relating to each stage collection plate is recovered using a suitable solvent and then analyzed, usually using HPLC to determine the amount of drug actually present.
By analyzing the amount of drug deposited on the various stages in this manner, it is then possible to calculate the FPD, FPF, MMAD and GSD. (Table 2)
In-vivo testing is performed to determine factors such as the pulmonary availability, clinical dose range, variability in patient response and side-effect profile. In order to evaluate the usefulness of in-vitro testing, it is important to determine if measurements conducted using inhaler devices in-vitro show any correlation with clinical effect in patients with asthma. This could be achieved by looking at the relationship between in-vitro measurements and both lung deposition (measured by gamma scintigraphy or by pharmacokinetic methods) and clinical effect.
Gamma Scintigraphy
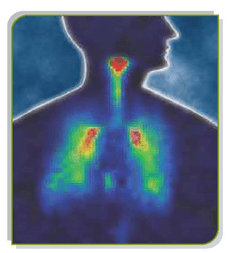
Gamma scintigraphy allows quantification of the percentage of the metered dose of drug that is deposited in the lungs. A gamma-ray emitting label is conjugated into the drug formulation and deposition of the inhaled drug is then followed by an external gamma camera.
Figure 4: Gamma Scintigraphy of radiolabelled drug
The aerosol is pre-labelled with a radioisotope which emits gamma-rays (technetium 99m). The drug labelled with radioisotope is then administered. The distribution of the drug plus isotope in the body after inhalation is then measured by using an external gamma camera. Actually, the gamma camera measures the gamma rays emitted by the radioisotope.
Using gamma-scintigraphy, it is possible to visualize the distribution of the inhaled drug in the lungs and oropharynx.
Pharmacokinetic Methods
Blood levels of the drug
Pharmacokinetic studies assess lung deposition by measuring systemic availability of the inhaled drug; this is done by administration of charcoal in order to prevent the absorption of the swallowed drug. This so-called charcoal-block method takes advantage of the fact that if the uptake of the oral and GI portions of an inhaled drug is blocked by activated charcoal, the amount of active drug reaching the systemic circulation equals the amount of active drug absorbed over the lung membrane. Thus, pharmacokinetic methods measure the absolute amount of drug taken up by the lungs.
Regional deposition, however, cannot be assessed by the pharmacokinetic method.
Urinary levels of drugs
The amount of salbutamol excreted in the urine over the first 30 min after an inhaled dose is an index of lung deposition. The 30-minutes urinary salbutamol method can be used for an inhalation period of up to 8 min to identify the relative bioavailability to the lung. Samples taken after this time period are affected by excretion of the oral absorbed fraction.
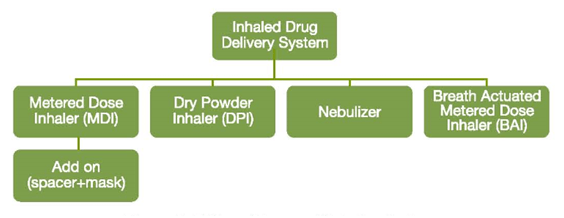
Classification of Inhaler Devices
Inhalation devices are classified into four types: pressurized metered dose inhalers (pMDIs), dry powder inhalers (DPIs), breath actuated inhalers (BAIs) and nebulizers.
The add-on devices which can be used along with the pMDIs are spacers and face mask. Each of the above-mentioned devices are equally effective if used correctly, but there are important differences in the ability of individual patients to use them as well as differences in cost, convenience, portability, and particle-generation characteristics.
Figure 5: Different types of inhaler devices
The effectiveness of a particular device depends on the ease of use and the patient’s ability to administer his or her own medication. It is, therefore, essential that the physician selects the right device for his patients. Generally, patients express preference for devices that are small, portable and easy to use while always ensuring that an accurate dose is dispensed.
From the manufacturer’s point of view, it is very important that inhaler devices are designed using a patient centered approach & that it meets the stringent manufacturing requirements in terms of its robustness, efficiency and precision whilst remaining environmental friendly.
References
- EurRespir J 2011; 37: 1308–1331
- Respir Care 2008; 53(6):699 –723
- Indian J Chest Dis Allied Sci 2008; 50: 209-219
- Respir Care 2005; 50(9):1177–1188
- Br J ClinPharmacol 2001, 51, 289-299