Composition
RETEFAST
Each single use vial contains:
Reteplase (Recombinant Tissue Plasminogen Activator) 18 mg, sterile lyophilized powder
Tranexamic Acid, Dipotassium Hydrogen Phosphate, Phosphoric acid, Sucrose, Polysorbate 80……….q.s.
Dosage Form
Sterile, white, lyophilized powder for intravenous bolus injection after reconstitution with sterile water for injection
Pharmacology
Reteplase is a non-glycosylated deletion mutein of tissue plasminogen activator, containing the kringle 2 and the protease domains of human tissue plasminogen activator. Reteplase contains 355 of the 527 amino acids of native tissue plasminogen activator (amino acids 1-3 and 176-527). Reteplase is produced by recombinant DNA technology in E. coli. The protein is isolated as inclusion bodies from E. coli, converted into its active form by an in vitro folding process and purified by chromatographic separation. The molecular weight of reteplase is 39,571 daltons.
Pharmacodynamics
Mechanism of Action
Reteplase is a recombinant plasminogen activator. It catalyzes the cleavage of endogenous plasminogen to generate plasmin. This plasminogenolysis takes place in the presence of fibrin. Plasmin in turn degrades fibrin, which is the main component of the matrix of thrombi, thereby exerting its thrombolytic action.
Clinical study on RETEFAST, done by the manufacturer, demonstrated efficacy by reducing mortality in patients with ST-segment elevation myocardial infarction (STEMI). RETEFAST also decreased the frequency of re-infarction, cardiac failure, cardiac arrhythmia and need for recanalization procedure which is comparable with earlier reported reteplase studies. There was also significant increase in left ventricular ejection fraction (LVEF) and 50% lowering of ST-segment in >50% of patients at 90th minute post dosing.
Pharmacokinetics
Based on the measurement of thrombolytic activity, reteplase is cleared from plasma at a rate of 250-450 mL/min, with an effective half-life of 13-16 minutes. Reteplase is primarily cleared by the liver and kidney.
Clinical Studies
Several clinical trials have demonstrated the efficacy and safety of reteplase in acute myocardial infarction.
RAPID Study (Reteplase Angiographic Phase II International Dose Finding Study)
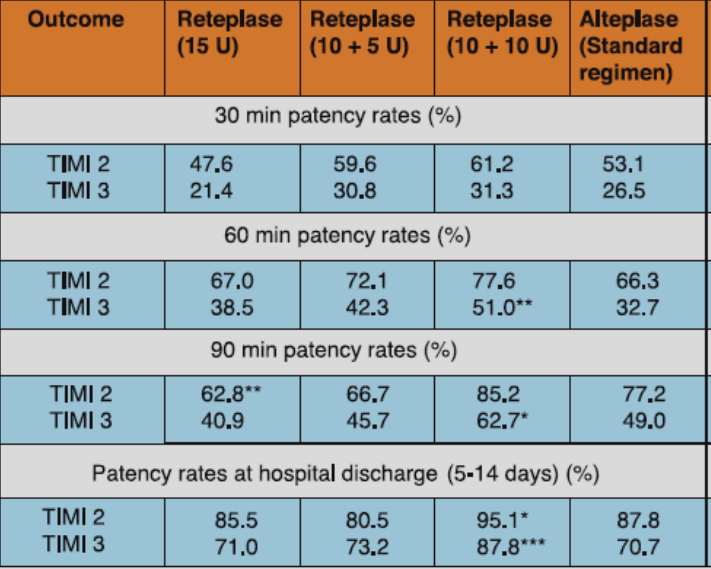
The RAPID study compared three doses of reteplase to alteplase in patients with acute myocardial infarction (AMI). A total of 606 patients presenting within 6 hours of symptom onset were randomized to either of the three doses of reteplase (10 + 10 U given 30 min apart, 15 U single bolus, or 10 + 5 U given 30 min apart) or to alteplase 100 mg administered over 3 hours. Aspirin 200-325 mg was given immediately before administration of the thrombolytic agent and it was later continued on a daily basis. Just before initiation of the thrombolytic drug, heparin was administered as 5000 U bolus followed by 1000 U/h for at least 24 hours. Coronary arteriography was performed at 30, 60 and 90 minutes after initiation of thrombolytic therapy and at hospital discharge. Left ventriculography was also performed after coronary arteriography.
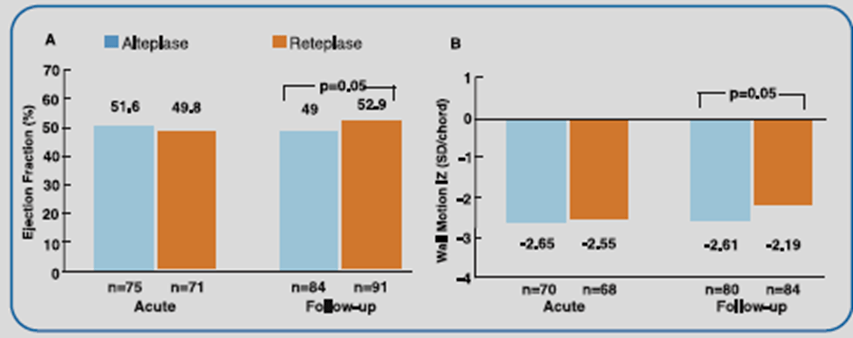
The best patency results were obtained with the 10 + 10 U reteplase dosing (Table 1). Reteplase 10 + 10 U also exhibited significantly favorable improvement in global ejection fraction and regional wall motion vs alteplase (Figure 1).
*p<0.05, **p<0.01, ***p<0.001 compared with alteplase.
Adapted from Ref. 1
Adapted from Ref. 1
RAPID II study (Reteplase versus Alteplase Patency Investigation During Acute Myocardial Infarction)
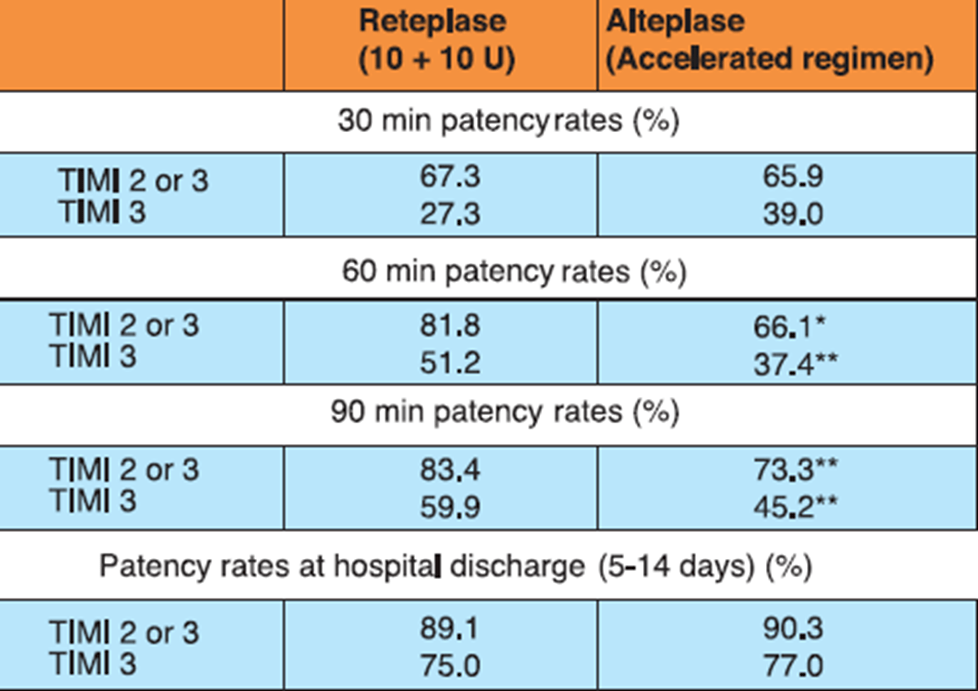
In the RAPID II study, 324 AMI patients who presented within 12 hours of symptom onset were randomized to receive either 10 + 10 U double bolus of reteplase given 30 minutes apart or accelerated (front-loaded) 90-minute alteplase regimen. Aspirin 160-320 mg/day (later continued on daily basis) and heparin 5000 U bolus (followed by 1000 U/h for at least 24 hours) were administered immediately before initiating thrombolytic therapy.
Reteplase achieved significantly higher rates of early reperfusion of the infarct-related coronary artery vs alteplase (Table 2). Besides, reteplase-treated patients had significantly lesser need of acute additional coronary interventions vs the alteplase-treated group (13.6% vs 26.5%; p<0.01). The 35-day mortality rates were also lower with reteplase vs alteplase (4.1% vs 8.4%; P=NS)
*p<0.05, **p<0.01
Adapted from Ref. 2
INJECT Study (International Joint Efficacy Comparison of Thrombolytics)
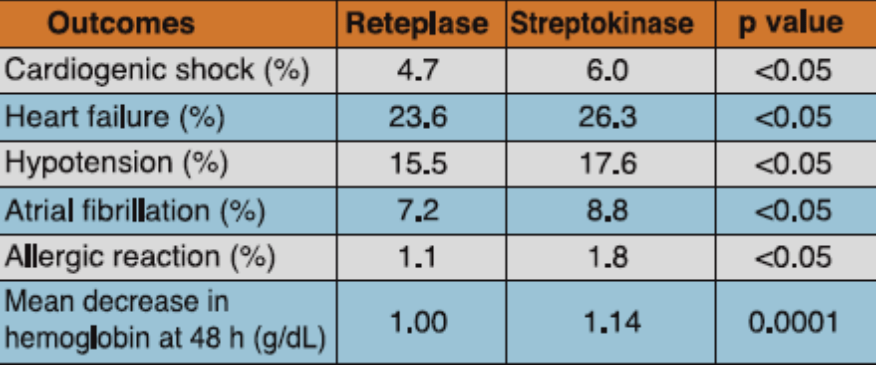
Reteplase (two boluses of 10 U given 30 min apart) was compared to streptokinase (1.5 MU administered intravenously over 60 minutes) in this double-blind, randomized, multicenter study, which evaluated the data of 6,010 patients admitted within 12 hours from the onset of symptoms of AMI. All patients received intravenous heparin for at least 24 hours. Patients were administered aspirin 250-320 mg initially and later maintained on 75-150 mg/day.
Mortality at day 35 was 8.90% in the reteplase group and 9.43% in the streptokinase group, a difference of -0.53%. At 6 month, mortality rate was 11.02% in the reteplase group and 12.05% in the streptokinase group, a difference of -1.03%.There were significantly lesser incidences of heart failure, hypotension, atrial fibrillation and cardiogenic shock among the patients treated with reteplase vs streptokinase. Besides, allergic reaction and mean decrease in hemoglobin at 48 hours was also significantly lesser with reteplase vs streptokinase (Table 3).
Adapted from Ref. 3
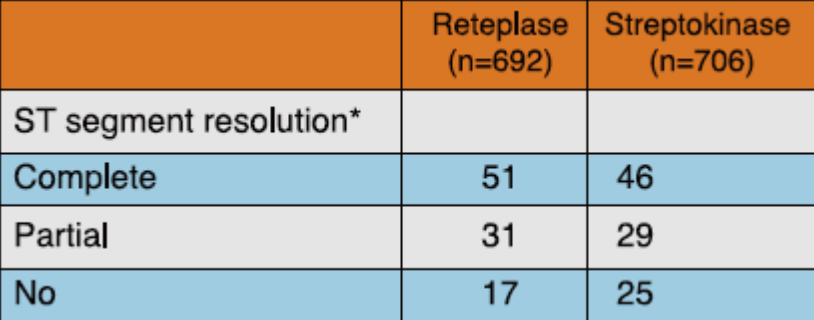
A sub-study conducted by INJECT investigators, in 1,398 patients presenting in < 6 h from onset of AMI showed that significantly more number of patients in the reteplase group had complete or partial ST-segment resolution at 3 hours as compared to those in the streptokinase group (82% vs 75% respectively, p= 0.006) (Table 4).
*p value = 0.006
Adapted from Ref. 4
MINAP (Myocardial Infarction in National Audit Project) Registry Analysis
This was an observational study, in which data of 49 017 episodes of STEMI recorded between 2005-2006 in the MINAP registry were analyzed. Of the total, 35 356 cases received treatment with a thrombolytic agent. Streptokinase was given to 20.2% of cases, alteplase in 3.7%, tenecteplase in 48.7% and reteplase in 22.6% of cases. Among the thrombolytic agents used, pre-hospital treatment (received in 14% of patients) was limited to reteplase and tenecteplase. Reinfarction (during hospital admission) status record was available for 22 391 (63.3%) patients and 6.5% (n=1360) had reinfarction. Reinfarction was defined as ischemic pain or other symptoms consistent with acute cardiac ischemia persisting until relieved by analgesia or nitrates, accompanied by new electrocardiographic changes (elevation or depression of the ST-segment or T wave changes) in the territory of the initial event. These features had to be accompanied by new elevation of creatine kinase or other markers of cardiac necrosis to more than the upper limit of normal or an increase to a value >50% greater than the previous value.
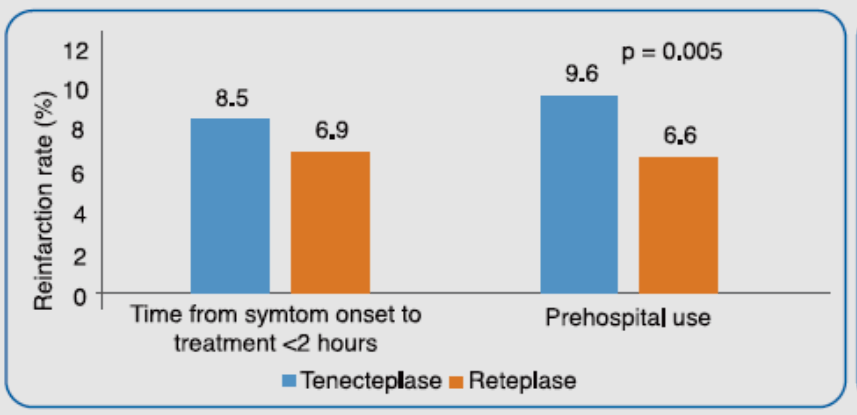
It was observed that both treatment with tenecteplase within 2 hours of onset of symptoms and pre-hospital treatment with tenecteplase was associated with higher incidence of reinfarction rates vs reteplase (Figure 2). For tenecteplase, prehospital treatment [Odds Ratio (OR): 1.44; p<0.001] and weight in the highest quartile (OR: 1.66; p = 0.003) were independent predictors of re-infarction. For reteplase, the independent predictors of reinfarction were chronic renal failure (OR: 2.60; p = 0.002) and previous myocardial infarction (OR: 2.74; p < 0.001).
Adapted from Ref. 5
Evidence with RETEFAST in Indian Patients
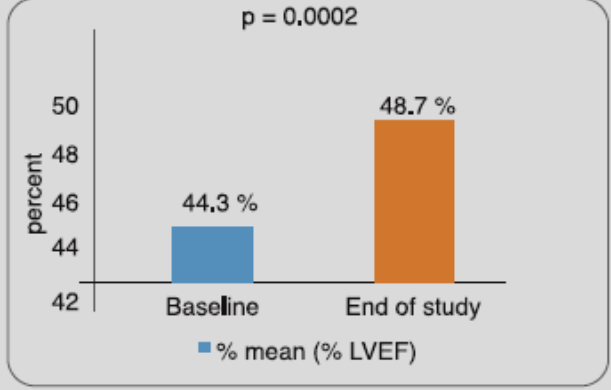
In a study done by the manufacturers of RETEFAST, 80 Indian STEMI patients, hospitalized within 6 hours of onset of symptoms, received a double bolus dose of reteplase (10 + 10 U, administered 30 minutes apart). Results showed that 51.25% patient achieved 50% lowering of ST-segment elevation at 90th minute post dosing. It further improved to 55% patients at 120 min and 73.75% patients at 6 hours post dosing. At Day-30, there was a significant improvement in left ventricular ejection fraction vs baseline (Figure 3).
Adapted from Ref. 6
Indications
Reteplase is indicated for the thrombolytic treatment in patients with persistent ST-segment elevation acute myocardial infarction (AMI) and/ or recent left bundle branch block. Treatment should be initiated as soon as possible after the onset of AMI.
Dosage and Administration
RETEFAST is for intravenous administration only.
RETEFAST is administered as two bolus injections of 10 units each. Each bolus is administered over 2 minutes. The second bolus is given 30 minutes after the first bolus injection.
No other medication should be injected or infused in the same intravenous line. No other medication should be added to the injection solution containing RETEFAST. There is no experience with patients receiving repeat courses of therapy with reteplase.
If RETEFAST is to be injected through an intravenous line containing heparin, a normal saline or 5% dextrose (D5W) solution should be flushed through the line prior to and following the RETEFAST injection.
Although the value of anticoagulants and antiplatelet drugs during and following administration of reteplase has not been studied, heparin has been administered concomitantly in most of the patients. Aspirin has been given either during and/or following heparin treatment.
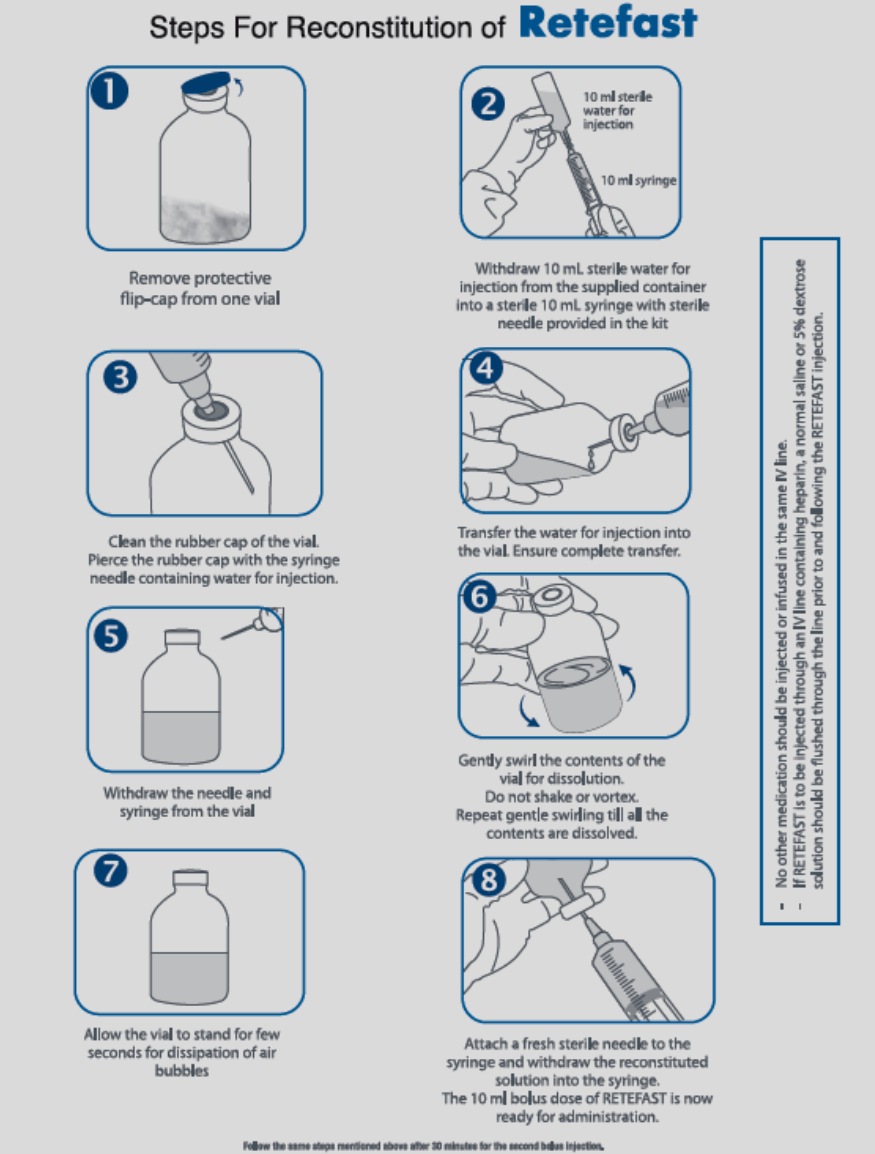
Reconstitution
Contraindications
- Reteplase should not be used in patients having hypersensitivity to reteplase, polysorbate 80 or any of the other ingredients of the product
- Because thrombolytic therapy increases the risk of bleeding, reteplase is contraindicated in the following situations:
- Active internal bleeding
- History of cerebrovascular accident
- Recent intracranial or intraspinal surgery or trauma
- Intracranial neoplasm, arteriovenous malformation, or aneurysm
- Known bleeding diathesis
- Severe uncontrolled hypertension
Warnings and Precautions
Drug Interactions
Interaction study reports with reteplase and medicinal products commonly administered in patients with AMI are not available. Heparin, vitamin K antagonists and medicinal product that alter platelet function (such as acetylsalicylic acid, dipyridamole and abciximab) may increase the risk of bleeding if administered prior to, during or after reteplase therapy.
Bleeding
Bleeding is the most common complication encountered during reteplase therapy. The sites of bleeding include both internal bleeding sites (intracranial, retroperitoneal, gastrointestinal, genitourinary, or respiratory) and superficial bleeding sites (venous cutdowns, arterial punctures, sites of recent surgical intervention). The concomitant use of heparin and other anticoagulants may contribute to bleeding.
Thrombolytic therapy requires careful attention to all potential bleeding sites (including catheter insertion sites, arterial and venous puncture sites, cutdown sites, and needle puncture sites).
Should an arterial puncture be necessary during the administration of reteplase, it is preferable to use an upper extremity vessel that is accessible to manual compression. Pressure should be applied for at least 30 minutes, a pressure dressing applied, and the puncture site checked frequently for evidence of bleeding.
Other venipunctures should be performed carefully and only as required.
In case of serious bleeding which is not controllable by local pressure, concomitant anticoagulant therapy should be terminated immediately. In addition, the second bolus of reteplase should not be given if serious bleeding occurs before it is administered.
Each patient considered for therapy with reteplase should be carefully evaluated and anticipated benefits weighed against the potential risks associated with therapy. In the following conditions, the potential risks of reteplase therapy may be increased and should be weighed against the anticipated benefits:
- Recent major surgery, e.g., coronary artery bypass graft, obstetrical delivery, organ biopsy
- Previous puncture of noncompressible vessels
- Cerebrovascular disease
- Recent gastrointestinal or genitourinary bleeding
- Hypertension: systolic blood pressure (BP) ≥180 mm Hg and/or diastolic BP ≥ 110 mm Hg
- Subacute bacterial endocarditis
- Hemostatic defects including those secondary to severe hepatic or renal disease
- Severe hepatic or renal dysfunction
- Diabetic hemorrhagic retinopathy or other hemorrhagic ophthalmic conditions
- Septic thrombophlebitis or occluded atrioventricular (AV) cannula at a seriously infected site
- Age over 75 years
- Patients currently receiving oral anticoagulants, e.g., warfarin sodium
- Any other condition in which bleeding constitutes a significant hazard or would be particularly difficult to manage because of its location
Arrhythmias
Coronary thrombolysis may result in arrhythmias associated with reperfusion. It is recommended that antiarrhythmic therapy for bradycardia and/or ventricular irritability be available when reteplase is administered.
Cholesterol Embolization
Cholesterol embolism has been reported rarely in patients treated with thrombolytic agents; the true incidence is unknown.
Renal Impairment
The potential risks of reteplase therapy may be increased in patients with severe renal dysfunction and should be weighed against the anticipated benefits.
Hepatic Impairment
The potential risks of reteplase therapy may be increased in patients with severe liver dysfunction and should be weighed against the anticipated benefits.
Pregnancy
Pregnancy Category C
Reteplase has been shown to have an abortifacient effect in rabbits when given in doses 3 times the human dose. Reproduction studies performed in rats at doses up to 15 times the human dose revealed no evidence of fetal anomalies; however, reteplase administered to pregnant rabbits resulted in hemorrhaging in the genital tract, leading to abortions in mid-gestation. There are no adequate and well controlled studies in pregnant women. Reteplase should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Lactation
It is not known whether reteplase is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when reteplase is administered to a nursing woman.
Undesirable Effects
Bleeding
The most commonly reported adverse drug reaction associated with reteplase treatment is hemorrhage, predominantly at the injection site.
Intracranial bleeding has been seen with reteplase therapy, which may be fatal in some cases. Systolic BP over 160 mm Hg before thrombolysis with reteplase was associated with greater risk for cerebral bleeding. As age increases the risk of intracranial bleeding and fatal intracranial bleeding increases.
Other Adverse Reactions
Patients administered reteplase as treatment for myocardial infarction have experienced many events which are frequent sequelae of myocardial infarction and may or may not be attributable to reteplase therapy. These events include cardiogenic shock, arrhythmias (e.g., sinus bradycardia, accelerated idioventricular rhythm, ventricular premature depolarizations, supraventricular tachycardia, ventricular tachycardia, ventricular fibrillation), AV block, pulmonary edema, heart failure, cardiac arrest, recurrent ischemia, reinfarction, myocardial rupture, mitral regurgitation, pericardial effusion, pericarditis, cardiac tamponade, venous thrombosis and embolism, and electromechanical dissociation. Allergic reactions were noted in clinical studies in a small number of patients.
Clinical Study with RETEFAST
Clinical study with RETEFAST, done by manufacturer, demonstrated it to be safe, well tolerated and easy to administer. The safety profile was comparable with earlier reported reteplase studies. Bleeding events with RETEFAST therapy were in 7.50%, which is comparable to earlier reported studies and only 2.50% patients, required blood transfusion. No stroke event was reported in this study. Other cardiac complications reported in the study were hypotension (12.50%), hypertension (10.00%), pericarditis (2.50%), bradycardia (8.75%) and thrombosis (2.50%).
Overdosage
In the event of over dosage one might expect depletion of fibrinogen and other blood coagulation components (e.g. coagulation factor V) with a consequent risk of bleeding.
Incompatibility
Heparin and reteplase are incompatible when combined in solution. Do not administer heparin and reteplase simultaneously in the same intravenous line.
If reteplase is to be injected through an intravenous line containing heparin, a normal saline or D5W solution should be flushed through the line prior to and following the reteplase injection.
Shelf-Life
2 years from the date of manufacturing.
Storage and Handling Instructions
Store at 2-8?C (36-46?F).
Prevent exposure to light.
Do not use beyond the expiration date.
Packaging Information
RETEFAST Kit: This kit contains -
- 2 single use RETEFAST vials. Each vial contains reteplase (recombinant tissue plasminogen activator) 18 mg, sterile lyophilized powder.
- 2 ampoules of 10 ml sterile water for injection IP for reconstitution
- 2 single use 10 ml sterile syringes
- 4 sterile needles
Last Updated: November 2015
Last Reviewed: November 2015
References
- Smalling RW, Bode C, Kalbfleisch J, et al. More rapid, complete, and stable coronary thrombolysis with bolus administration of reteplase compared with alteplase infusion in acute myocardial infarction. Circulation. 1995;91:2725-32.
- Bode C, Smalling RW, Berg G, et al. Randomized comparison of coronary thrombolysis achieved with double-bolus reteplase (recombinant plasminogen activator) and front-loaded, accelerated alteplase (recombinant tissue plasminogen activator) in patients with acute myocardial infarction. Circulation. 1996;94:891-8.
- INJECT Study Group. Randomised, double-blind comparison of reteplase double-bolus administration with streptokinase in acute myocardial infarction (INJECT): trial to investigate equivalence. Lancet. 1995;346:329-36.
- Schroder R, et al. Extent of Early ST Segment Elevation Resolution: A Strong Predictor of Outcome in Patients With Acute Myocardial Infarction and a Sensitive Measure to Compare Thrombolytic Regimens. J Am Coll Cardiol 1995;26:1657-64.
- Horne S, et al. The impact of pre-hospital thrombolytic treatment on re-infarction rates: analysis of the Myocardial Infarction National Audit Project (MINAP). Heart 2009;95:559-63.
- Shah K, et al. Clinical retrospective and prospective evaluation of efficacy and safety of reteplase in STEMI patients. Indian Medical Gazette 2012:479-87