New Revolizer™
A Revolution


Dry powder inhalers (DPIs) are cost-effective, simple-to-use and environmentally preferred devices for pulmonary drug delivery.
The revolizer is a novel DPI device, which is the result of a design and development programme to address the critical requirements of an ideal inhalation device. It provides accurate dosing and excellent lung deposition even at low inspiratory flow rates combined with ease of use. It provides an improved respirable fraction while adding style and modern design to give patients a revolution in inhalation therapy.
The revolizer is an extremely simple device. The user opens the device, inserts the Rotacap, shuts the device and inhales.
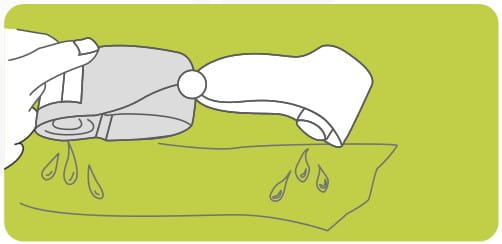
Monitor Drug Transfer
Revolizer gives the user 3 key indications to monitor drug administration

Parts of the New revolizer
The revolizer consists of the mouthpiece, the rotacap chamber and the base.
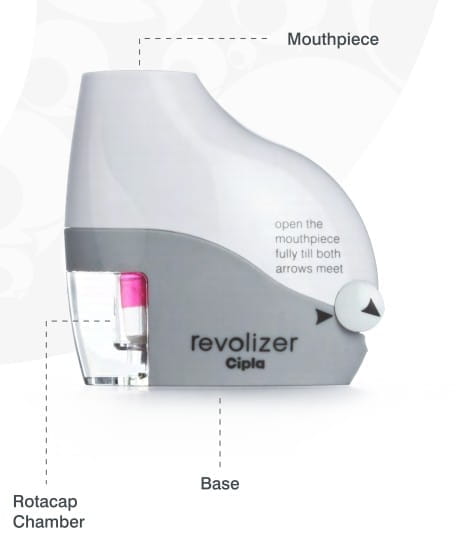
The revolizer consists of the mouthpiece, the rotacap chamber and the base.
The Mouthpiece
It is designed to provide a good grip of the mouth on the device.

The mouthpiece is shaped to provide a wide opening to the mouth. This forces the tongue out of the way creating an unobstructed oral cavity through which the drug can travel. It includes a mesh, which ensures that, even in the most unlikely situation of the Rotacap breaking into pieces, the fragments will not be inhaled.
The Rotacap Chamber
It holds the medication Rotacap during inhalation. The design of the Rotacap chamber and the baffles inside ensures maximum drug delivery to the targeted site of action - the lungs.

Its size is optimized to oscillate the Rotacap and ensure rapid and complete evacuation of the medication.
The Base
It houses the spike assembly, which gets activated when the revolizer is opened or closed.

The pins are made of high grade, rust proof, stainless steel of SS304 specification which exhibits excellent resistance to corrosion and oxidation.
The spike enters the capsule chamber through the guides provided and pierces two holes in the capsule from which the medication is released for inhalation.
- The principle of operation in revolizer is vibrational motion.
- The device is designed in such a way that the internal geometry of the capsule chamber maximizes the difference in pressure thereby forcing the drug to be evacuated into the air flow.
- The internal geometry of capsule chamber is configured in such a way that there is enhanced mechanical agitation of the capsule which would aid deaggregation of the lactose and drug.
- Mouthpiece of the device has a tapered end which causes increased capsule turbulence, thus aids in having an equal distribution of the drug loaded powder in the inhaled air.
- Narrow air entry at the base of the capsule chamber causes lot of pressure variation in the capsule chamber.

The mouthpiece of the new revolizer has been modified to a long and narrow straight air path with 1° taper from the top to bottom for better demolding of the part leading to gradual increase in the air velocity creating a laminar air flow resulting in increase turbulence of the capsule.
The size of the mesh holes in the new revolizer is made finer. It has also been modified to capture the fragments of the capsule if the capsule breaks.
The interior geometry of the capsule chamber is tubular in shape. The device is designed in such a way that the internal geometry of the capsule chamber maximizes the difference in pressure thereby forcing the drug to be evacuated into the airflow.
Further there is enhanced mechanical agitation of the capsule which would aid deaggregation of the lactose and drug.
The exclusion of air vents in the mouth piece of the new revolizer provides 100% air flow in the device thus leading to increased turbulence of the capsule which has also helped in reducing the flow rate corresponding to 4 kPa pressure drop of the device from ∼50 L/min to ∼42 L/min.
'O' ring removed in the new design for better snap fitment and has thus helped in reducing 1 component for assembly.
The luvers are provided at the base for water to drain quickly after washing the revolizer.
The new revolizer can be held in any position for powder to evacuate from the capsule. (The existing revolizer required to be held in horizontal position for evacuation of powder from the capsule). Thus the new revolizer is easier & more comfortable to use.
The new revolizer stored at high temperature of 40°C and even at low temperature of 2-8°C remains stable.
The revolizer has been designed to be robust enough to withstand the impact of dropping. Even in such conditions, it remains fully functional without any damage to the external casework.
The revolizer has been assessed for pharmaceutical performance under standard laboratory conditions using various in vitro techniques.
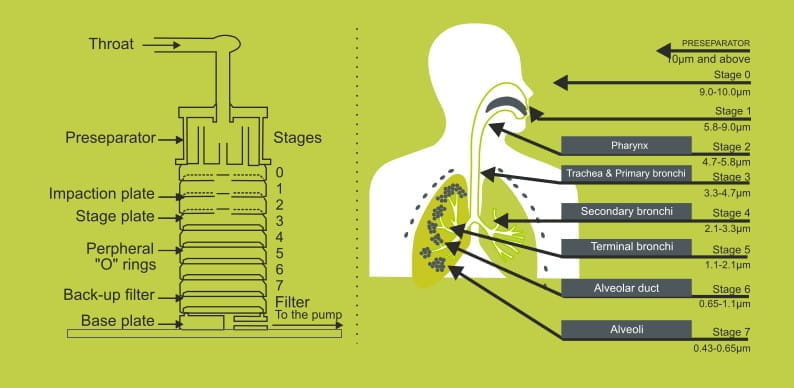
It is generally accepted that in order for a particle to reach the site of action within the respiratory tract, it must have an aerodynamic particle size between 0.5-10 microns, more preferably 1-5 microns. Fine particle dose (FPD) is the dose of particles having aerodynamic particle size less than 5 microns. The higher the FPD an inhaler delivers, the more of the drug will reach the site of action within the lungs.
Fine particle mass (FPM) simply represents drug deposition by mass of percentage and can be measured using a cascade impactor. The impactor consists of several stages which are arranged such that the size of particles they capture reduces successively from the top to the bottom stage. Each stage is designed to collect particles larger than its effective cut-off diameter. According to the Pharmacopoeia, the metered dose is extracted from the inhaler by drawing 4 litres of air at a constant flow rate which corresponds to 4 kPa pressure drop across the inhaler. This pressure drop represents the average inspiratory effort of asthmatics. The air stream will carry the drug particles from the inhaler into the impactor. The amount of drug deposited onto each stage is analysed using validated high performance liquid chromatography (HPLC) methods.
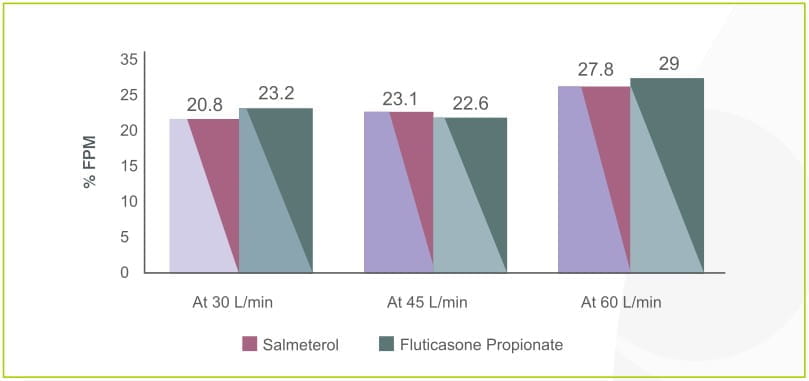
The percentage fine particle mass of salmeterol and fluticasone propionate in Seroflo Rotacaps with revolizer has been studied using the Cascade impactor at various flow rates of 30 L/min, 45 L/min and 60 L/min. Revolizer showed a high drug deposition of both the drugs at 60 L/min and even at low flow rates of 30 L/min.
%Fine Particle Mass (FPM) using Cascade Impactor: Seroflo 250 Rotacaps in Revolizer, Batch no. : D11168
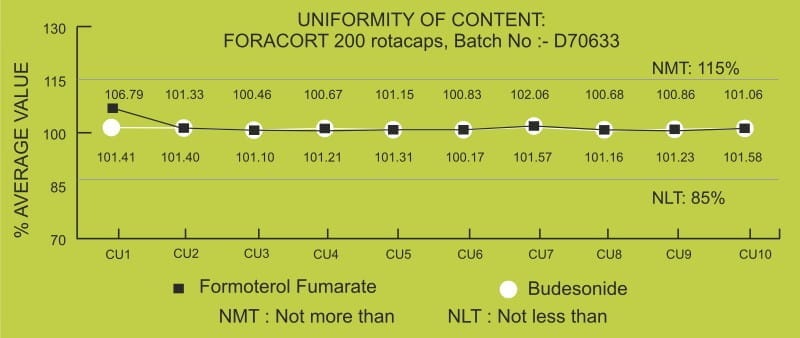
The performance of the revolizer also relies on the Rotacap which contains the drug. The Rotacap undergoes stringent quality tests so as to deliver consistent dosing. The content uniformity test gives an assurance of consistent drug content across all Rotacaps. Foracort Rotacaps when tested for budesonide and formoterol content were found to be well within the required limits.
The percentage fine particle mass of budesonide and formoterol in Foracort 200 Rotacaps with revolizer and Budamate 200 Transcaps with lupihaler has been studied using the Cascade impactor at various flow rates of 30 L/min, 45 L/min and 60L/min. Revolizer showed a high drug deposition at 60 L/min and even at low flow rates of 30 L/min.
%Fine Particle Mass (Fpm) At Variable Flow Rate: BUDESONIDE
Foracort 200 Rotacaps In New Revolizer Vs
Budamate 200 Transcaps In Lupihaler
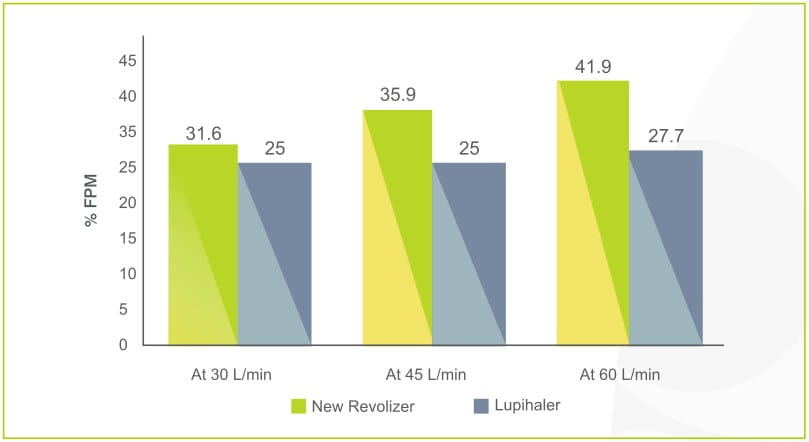
The % FPM values of Budesonide for Foracort 200 Rotacaps in revolizer was higher as compared to % FPM values of Budesonide for Budamate 200 Transcaps in lupihaler device.
%Fine particle mass (fpm) at variable flow rate: formoterol fumarate dihydrate foracort 200 rotacaps in new revolizer
vs
budamate 200 transcaps in lupihaler
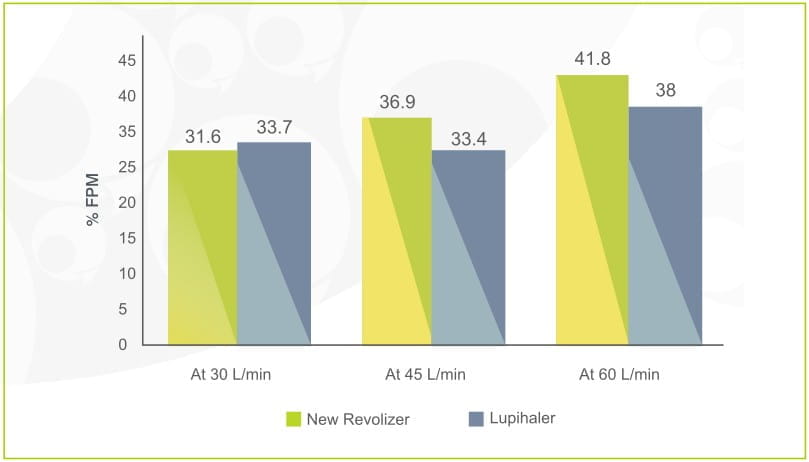
The % FPM values of Formoterol for Foracort 200 rotacaps in revolizer was comparable to % FPM values of Formoterol for Budamate 200 Transcaps in lupihaler device.
The optimal management of obstructive airway disease depends on adequate drug delivery to the airways by inhaler devices for which competent inhaler technique is essential. Difficulties using inhaler devices can lead to inadequate symptom control and exacerbations which increase the cost of therapy and negatively impact the quality of life. Further, in any chronic disease, compliance of patients with their prescribed treatments will inevitably depend on day-to-day convenience and simplicity of drug administration. In addition to receiving an accurately targeted dose of drug, the patient must like the inhaler. Therefore, the confidence, usability, preference and satisfaction of patients in use of inhalers are important when prescribing inhalers to the patients and should be given careful consideration.
Hence this study was conducted to assess the confidence, usability, preference and satisfaction on use of revolizer in healthy volunteers and in subjects with mild asthma or COPD.

- iprefer was the 1st study which was conducted on an indigenously developed device (revolizer) on Indian patients.
- This study was accepted and presented at European Respiratory Society (ERS), 2010
Objective
The study objectives were to assess the confidence, usability, preference and satisfaction on use of revolizer in healthy volunteers and in subjects with mild asthma or COPD.
Study Design
- iprefer study was an open label, prospective, multicentre study which was carried out in 50 healthy volunteers and in 50 subjects with mild asthma or COPD.
- During visit 1, inhaler technique was explained and demonstrated by the investigator, after which participants then repeated the procedure until they achieved three consecutive correct attempts. Following this they filled a questionnaire.
- During visit 2 (two days later) participants made a single attempt before receiving demonstration from the investigator. They then repeated the procedure until three consecutive correct attempts were made and then filled the questionnaire.
Results
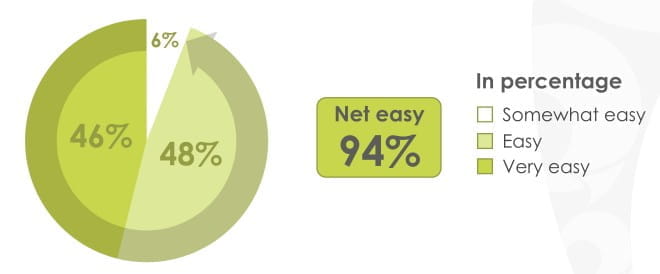
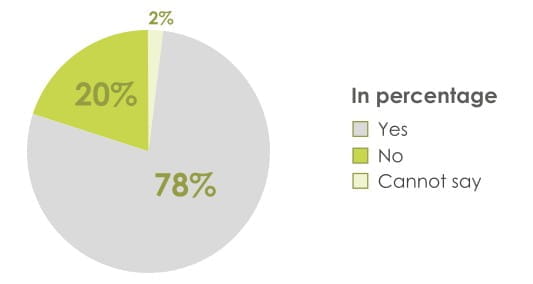
84% of patients found the revolizer easy to open & prepare for inhalation
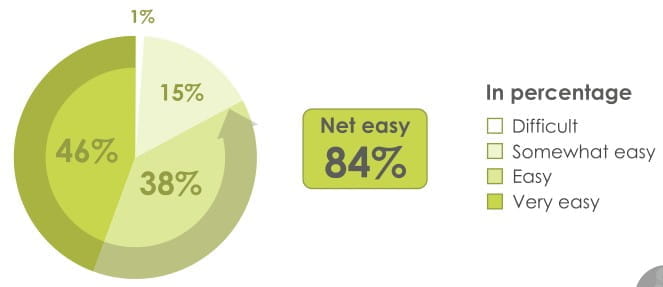
86% of patients found the mouthpiece of the revolizer comfortable to hold
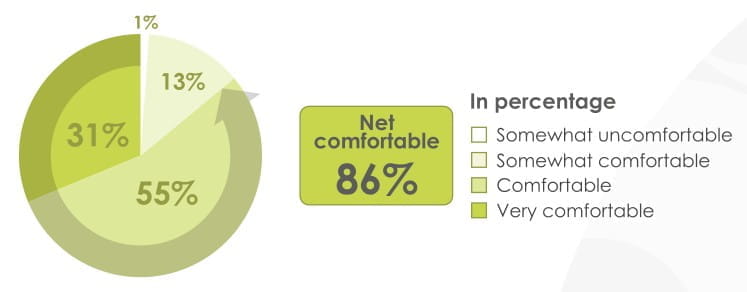
94% of patients found the revolizer easy to use
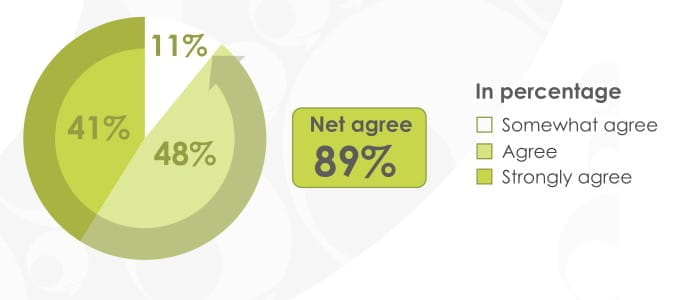
89% of patients found it was easy to remember how to use the revolizer
74% Patients agreed revolizer can be used quickly in case of emergency
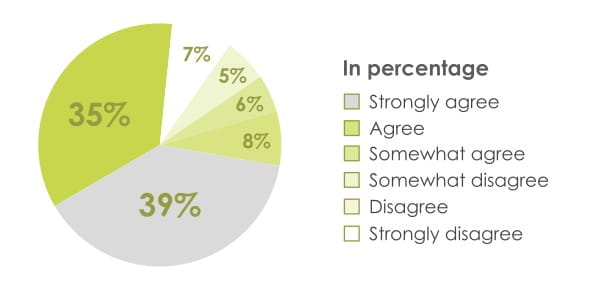
78% of patients preferred revolizer over their current device
Other Major Results of the iprefer Study
- Patients took 3 minutes to use the revolizer correctly for 3 consecutive attempts.
- On an average patients made 2 attempts to learn the correct use of revolizer.
- 94% of patients felt confident about using the revolizer.
- 96% of patients found it easy to understand how to use the revolizer.
- 92% of patients found it was easy to keep the revolizer clean.
- After using the revolizer, 98% of patients felt that they used the device correctly.
Errors Made by Patients in the Study
The errors are being mentioned in the order of steps for using the revolizer.
Step - 1: Arrows did not meet.
Step - 2: Did not insert the rotocap transparent side into the capsule chamber.
Step - 3: Did not lock the revolizer properly.
Step - 4: Did not exhale properly prior to inhalation.
Step - 5: Did not grip the mouthpiece.
Step - 6: Inhalation was slow.
Step - 7: Did not hold the breath after inhalation.
Step - 8: Did not discard the used rotacap.
The most frequent error observed was in step 4 (exhalation prior to inhalation through the device is required). The second most frequent error was in step 6 (rapid and deep inhalation through the device is required).
The total number of attempts required to achieve the goal and the critical errors in visit 2 decreased as compared to visit 1.
The errors are purposefully mentioned here so that the patient remains fully aware about the most common errors being made while using revolizer, and he/she will be extra careful while using revolizer not repeating the above mentioned common errors.
Conclusion
The results of the iprefer study show that revolizer is easy to use and remember. The participants were confident in their ability to use the device. They preferred the device and showed overall satisfaction. Thus, revolizer represents a reasonable alternative to other available dry powder inhalers.
Adherence to treatment is a key issue in long-term management of both asthma and COPD. One of the factor that determines adherence is the ease of use of device. Studies have shown that patients prefer devices which are easy to use and require minimal training and this in turn helps ensure adherence to the prescribed regimen.*
Since the revolizer is easy to use, convenient, portable, stylish, user friendly; this would lead to improved adherence on the part of patient which would eventually lead to a better control of disease. This would significantly improve patients quality of life.
- Does not require coordination between inhalation and actuation
- Easy to learn, remember and teach how to use correctly
- High drug deposition across flow rates
- Accurate, consistent, and effective in delivering medication to the lungs even at low inspiratory flow rates
- Ergonomic design
- Portable - with the pouch snugly fits into the patient's pocket
- Patient feedback due to the taste of lactose in the mouth
- Transparency helps provide visual confirmation of the dose being administered
- Robust
- Visually appealing to the patient
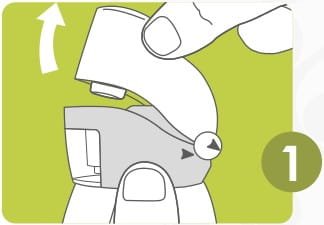
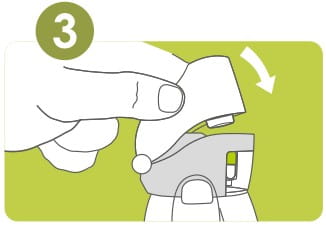
To open, hold the revolizer at the base with one hand.
Pull back the mouthpiece as shown till both arrows meet.
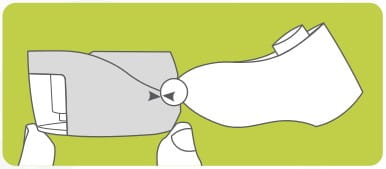
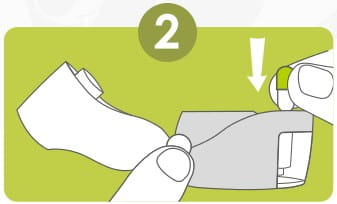
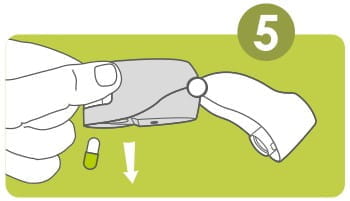
Remove a Rotacap from its bottle. Insert the Rotacap into the Rotacap chamber with the transparent end facing down as shown.
Close the mouthpiece firmly. A 'click' sound will indicate proper closing of the revolizer.
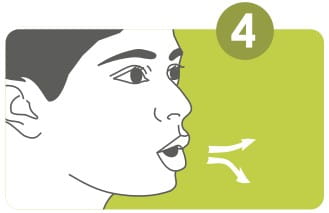
Breathe out fully, through your mouth. Position the mouthpiece between your teeth and close your lips tightly around it.
Sit or stand upright, keep your head straight, and breathe in through your mouth as rapidly and deeply as you can. When done correctly, you will hear the Rotacap vibrating inside the revolizer
Remove the revolizer from your mouth and hold your breath for about 10 seconds or for as long as it is comfortable. It is important to inhale all the powder in the Rotacap chamber. You may need to repeat step 4 in case some powder remains in the Rotacap chamber.
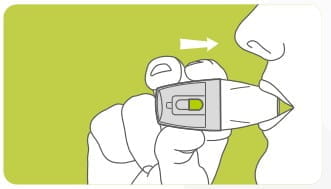
After every use, open the mouthpiece (till both arrows meet) and discard the empty Rotacap. Close the mouthpiece and store the revolizer in the convenient carry pouch provided.
For your next use, take a new Rotacap and follow step 1 to 5.
- You must remember to clean the revolizer at least once a week to ensure smooth functioning.
- Open the mouthpiece (till both the arrows meet) of the revolizer.
- If there is an empty Rotacap in the chamber, remove it.
- Rinse the mouthpiece and Rotacap chamber in clean running water.
- Shake well to remove excess water and leave to air dry. This can take upto 6 hours, so preferably clean the revolizer and leave to dry overnight. Do not expose the revolizer to heat.
- Store the revolizer along with the Rotacaps bottle in the convenient carry pouch provided.
- Keep the Rotacap bottle tightly closed to avoid exposure to moisture.
- Keep the revolizer clean and dry at all times. Never use the revolizer when it is wet.
- It is recommended to use a new revolizer every 6 months.
- Use only as directed by your doctor. If the recommended dose not provide relief from symptoms or if symptoms become worse, consult your doctor.
- Open the Rotacap bottle just prior to use.
- At times a fine layer of powder may remain in the empty Rotacap after use. This is normal and does not alter the effect of the medicine.
CAUTION: DO NOT PUSH ANY CLOTH OR INSTRUMENT INTO THE MOUTHPIECE OF THE REVOLIZER AS THIS MAY CAUSE DAMAGE