Rivazest (Rivaroxaban) Monograph
Background on Novel Oral Anticoagulants
Treatment of venous and arterial thrombotic diseases is a major medical challenge, and development of anticoagulant drugs represents a revolution in medicine. Thromboembolic diseases are of major clinical concern because of their high prevalence and consequences that are often fatal. Venous thromboembolism (VTE) including deep vein thrombosis (DVT) and pulmonary embolism (PE) is the third most prevalent cause of vascular death after coronary heart disease and stroke.1 The mainstay of thrombosis treatment is anticoagulation.2 The route of administration of anticoagulant drugs can be either parenteral or oral.1
Past 60 years, vitamin K antagonists (VKAs; coumarin derivative-warfarin and acenocoumarol) have been the only oral anticoagulants (OACs) used in clinical practice. Now, new drugs with anticoagulants effects referred to as non-vitamin K antagonist oral anticoagulants (NOACs) have recently been discovered. Compared to VKAs, this new generation NOACs has more predictable anticoagulant responses and are selective for one specific coagulation factor, either thrombin or activated factor Xa. They are effective in the following conditions:1
- Treatment of DVT and PE.
- Prevention of recurrent DVT and PE.
- Prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation (NVAF) with one or more risk factors.
- Prevention of VTE in patients after elective hip or knee arthroplasty.
The dose of VKA is determined on an individual basis but novel NOACs are administered in fixed doses, except when a patient has a functional disorder of the liver or kidney.1 Several guidelines suggest the use of direct NOACs such as rivaroxaban over vitamin K antagonists such as warfarin. Anticoagulation therapy must be administered for 3 months or longer, depending on the balance between the risk of recurrent VTE and the risk of bleeding. In patients without reversible risk factors, the risk of recurrent VTE is as much as 10% in the first year if anticoagulation therapy is stopped. Patients in whom thrombosis was triggered by nonsurgical risk factors or with persistent risk factors are at higher risk for recurrence than are patients with postoperative thrombosis. In addition, because of overlapping risk factors, patients with VTE are at increased risk for arterial thrombotic events, including myocardial infarction (MI), stroke and vascular death. Although extended anticoagulation therapy is effective for the prevention of recurrent VTE, concern about bleeding often leads to a reluctance to continue anticoagulant treatment beyond 6 to 12 months. In case of extending the anticoagulant therapy, the risk of bleeding can be reduced by using the use of lower-dose anticoagulant therapy and the use of aspirin in place of an anticoagulant agent.2
Rivaroxaban – Scientific Information
Rivaroxaban is the first NOAC that received European approval for the prevention of atherothrombotic events in patients with acute coronary syndrome. It is a novel oral anticoagulant that is approved in many countries worldwide for1,3
- Treatment of DVT and PE.
- Prevention of recurrent DVT an PE.
- To reduce the risk of stroke or systemic embolism in patients with NVAF and one or more risk factors for stroke.
- Prevention of VTE after elective hip or knee replacement surgery.
- In combination with acetylsalicylic acid (ASA) for the prevention of atherothrombotic events in patients with acute coronary syndrome (ACS) who have elevated cardiac biomarkers.
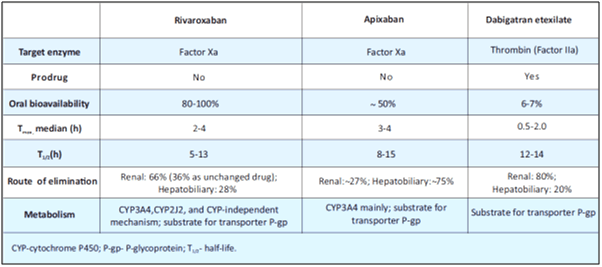
Compared to VKAs, rivaroxaban shows rapid onset of action, predictable pharmacokinetics and pharmacodynamics, no dietary interactions, few drug interactions and does not require routine coagulation monitoring (see Table 1). According to the 2012 updated guidelines for stroke prevention in AF, from the European Society of Cardiology (ESC), rivaroxaban is recommended over the use of VKAs.3
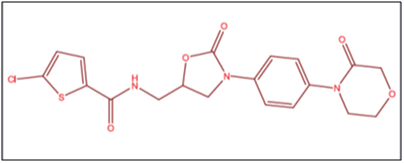
Common Name: Rivaroxaban.
Chemical Name: 5-chloro-N-[[(5S)-2-oxo-3-[4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl]methyl]thiophene 2-carboxamide).4
Molecular Formula: C19H18Cl N3O5S
Molecular Mass: 435.89
Structural Formula: Figure 1 depicts the structure of rivaroxaban
Physicochemical Properties
Rivaroxaban is a pure (S)-enantiomer. It is an odourless, non-hygroscopic and white to yellowish powder. Rivaroxaban is practically insoluble in water (7 mg/L, pure water) and remains so in aqueous acidic medium (5 mg/L, in 0.1 M and 0.01 M hydrochloric acid) or buffer systems, pH 3 to 9 (5 mg/L).5
Mechanism of Action
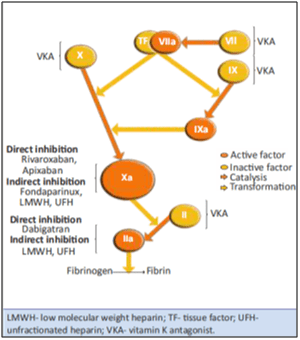
The central role in the cascade of blood coagulation is the activation of Factor-X to Factor-Xa (FXa) via the intrinsic and extrinsic pathway. Factor-Xa then directly converts prothrombin to thrombin through the prothrombinase complex and finally this leads to fibrin clot formation and activation of platelets by thrombin. Selective inhibitors of FXa can terminate the amplified burst of thrombin generation, thereby diminishing thrombin-mediated activation of coagulation.
Rivaroxaban is a highly selective first oral drug that directly inhibits Factor Xa with high oral bioavailability (see Fig. 2). It is an oxazolidinone derivative that binds directly and reversibly to Factor Xa via the S1 and S4 pockets. Rivaroxaban competitively inhibits Factor Xa and is >10,000-fold more selective for Factor-Xa than other related serine proteases. It does not require cofactors such as antithrombin to exert its anticoagulant effect. Unlike indirect Factor Xa inhibitors, rivaroxaban inhibits both free and clot-bound Factor-Xa, as well as prothrombinase activity, thereby prolonging clotting times. Rivaroxaban has no direct effect on platelet aggregation, but indirectly inhibits platelet aggregation induced by thrombin. By inhibiting FXa, rivaroxaban decreases thrombin generation.
Clinical Evidence on Rivaroxaban
A. Rivaroxaban in Prevention of Stroke and Systemic Embolism in Patients with NVAF
ROCKET AF Trial
Objective
Rocket AF trial is a prospective, randomised, double-blind, double-dummy, parallel-group, multicentre, pivotal clinical study that compared the efficacy and safety of 20 mg oral rivaroxaban once-daily (OD) with dose-adjusted warfarin in patients with nonvalvular atrial fibrillation (NVAF) who are at risk of stroke or systemic embolism. The aim of the study was to demonstrate that rivaroxaban was non-inferior to warfarin in reducing the occurrence of the composite endpoint of stroke and systemic embolism. If non-inferiority was shown, a pre-specified step-wise multiple testing procedure was undertaken to determine whether rivaroxaban was superior to warfarin in primary and secondary endpoints.8,9
Design and Methods
- A total of 14,264 patients with a mean age of 71 years and a mean CHADS2 score of 3.5 who were at increased risk of stroke were included.
- Patients were randomized to 20 mg OD rivaroxaban (15 mg in patients with moderate renal impairment at screening) or to dose-adjusted warfarin, titrated to an INR of 2.0 to 3.0.
- The primary efficacy end point was the composite of stroke (ischemic or haemorrhagic) and systemic embolism.
- Secondary efficacy end points included a composite of stroke, systemic embolism or death from cardiovascular causes; a composite of stroke, systemic embolism, death from cardiovascular causes, or MI; and individual components of the composite end points.
- The principal safety end point was a composite of major and non-major clinically relevant bleeding events.
Results
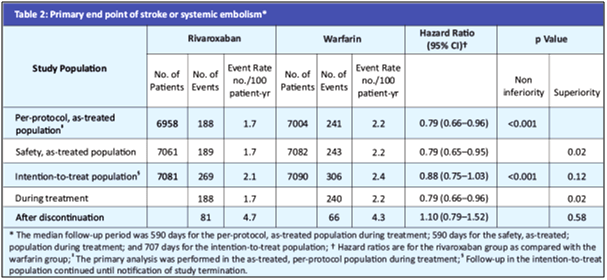
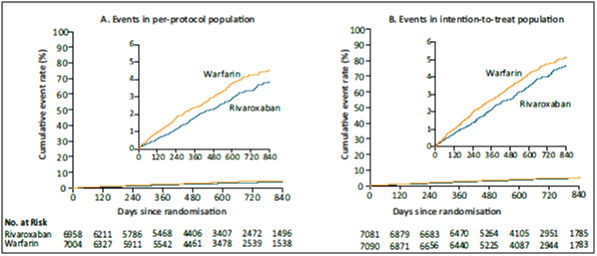
- In the primary efficacy endpoint, the primary end point occurred in 188 patients in the rivaroxaban group vs. 241 in the warfarin group (1.7% vs. 2.2% per year) (HR in the rivaroxaban group, 0.79; 95% confidence interval [CI], 0.66 to 0.96; p<0.001 for noninferiority) (see Table 2 and Fig. 3A).
- In the as-treated safety population, primary events occurred in 189 patients in the rivaroxaban group vs. 243 patients in the warfarin group (1.7% vs. 2.2% per year) (HR, 0.79; 95% CI, 0.65 to 0.95; p=0.01 for superiority).
- In the intention-to-treat analysis, the primary end point occurred in 269 patients in the rivaroxaban group vs. 306 patients in the warfarin group (2.1% vs. 2.4% per year) (HR, 0.88; 95% CI, 0.74 to 1.03; p<0.001 for noninferiority; p=0.12 for superiority, see Fig. 3B).
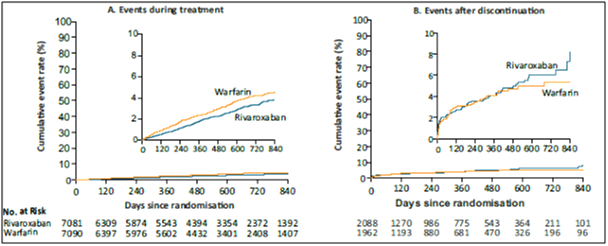
- During treatment in the intention-to-treat population, patients in the rivaroxaban group had a lower rate of stroke or systemic embolism (188 events, 1.7% per year) than those in the warfarin group (240 events, 2.2% per year) (p=0.02) (Fig. 4).
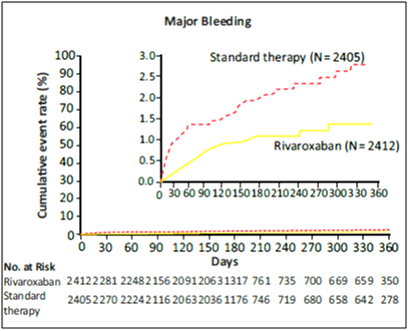
- Major and non-major clinically relevant bleeding occurred in 1475 patients in the rivaroxaban group vs. 1449 in the warfarin group (14.9% vs. 14.5% per year) (HR, 1.03; 95% CI, 0.96 to 1.11; p=0.44), with significant reductions in intracranial haemorrhage (0.5% vs. 0.7%, p=0.02) and fatal bleeding (0.2% vs. 0.5%, p=0.003) in the rivaroxaban group.
- The rates of secondary efficacy outcomes in the as-treated safety population:
o During treatment, MI occurred in 101 patients in the rivaroxaban group and 126 patients in the warfarin group (0.9% and 1.1% per year, respectively; hazard ratio in the rivaroxaban group, 0.81; 95% CI, 0.63 to 1.06; p=0.12).
o There were 208 deaths in the rivaroxaban group and 250 deaths in the warfarin group (1.9% and 2.2% per year, respectively; hazard ratio, 0.85; 95% CI, 0.70 to 1.02; p=0.07).
o In the intention-to-treat analysis throughout the trial, there were 582 deaths in the rivaroxaban group and 632 deaths in the warfarin group (4.5% and 4.9% per year, respectively; HR, 0.92; 95% CI, 0.82 to 1.03; p=0.15).
Conclusion
The authors concluded that rivaroxaban was noninferior to warfarin for the prevention of stroke or systemic embolism. There was no significant difference between rivaroxaban and warfarin in the risk of major bleeding, but intracranial and fatal bleeding occurred less frequently in the rivaroxaban group.8,9
Effectiveness and Safety of Low-Dose Rivaroxaban in Asians with NVAF
Background
Rivaroxaban (20 mg/15 mg OD) is an effective and safe alternative to warfarin for prevention of stroke in patients with NVAF. Low-dose rivaroxaban (15 mg/10 mg OD) has been only approved for NVAF patients in Japan and Taiwan, although its effectiveness and safety at low doses remain unclear among Asians with NVAF.11
Objective
The present study compared the effectiveness and safety of low dose rivaroxaban to warfarin among Asians with NVAF.
Design and Methods
- This study enrolled a total of 14,971, 11,029, and 16,000 consecutive patients taking rivaroxaban 15mg, rivaroxaban 10mg, and warfarin, respectively.
- The medium follow-up period was 1.2, 1.0 and 1.4 years for rivaroxaban 15 mg, rivaroxaban 10 mg and warfarin groups, respectively.
Results
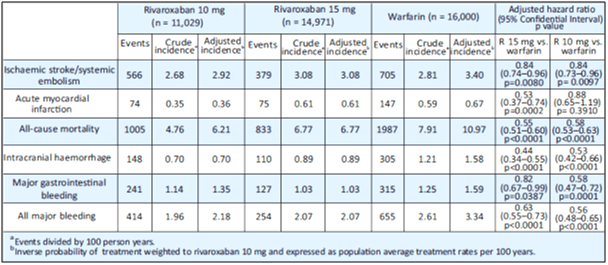
- Patients in both rivaroxaban groups had a lower risk of ICH, major GIB and all major bleeding compared with patients in the warfarin group before as well as after propensity score weighting (all p<0.05 after propensity score weighting, respectively) (see Table 3).
- After the weighting adjustment, the annual incidence rates of IS/SE of the two rivaroxaban groups (2.91%/year and 3.08%/year for the rivaroxaban 15 mg and 10 mg groups, respectively) were significantly lower than that of the warfarin group (3.40%/year; p=0.0080, p=0.0097, respectively).
- Patients in the 15 mg rivaroxaban group showed a lower risk of AMI compared with patients in the warfarin group, before as well as after the weighting adjustment (0.36%/year vs. 0.67%/year).
- Both doses of rivaroxaban were associated with a lower risk of IS/SE compared with warfarin in subgroups with <75 years of age, higher CHA2DS2-VASc and HAS-BLED scores (Supplemental Fig. II). Rivaroxaban 15 mg showed a lower risk of AMI compared to warfarin in most subgroups.
- Patients in both rivaroxaban groups showed a lower risk of ICH and all major bleeding compared with patients in the warfarin group in most subgroups.
Conclusion
Both the low doses of rivaroxaban were associated with a lower risk of ischemic stroke/systemic embolism, intracranial haemorrhage, gastrointestinal bleeding, all major bleeding and all-cause mortality compared with warfarin in Asian NVAF patients. The 15 mg rivaroxaban dose was associated with a lower risk of AMI compared to warfarin.
Use of Rivaroxaban vs. Dabigatran in NVAF
Background and Objective
Direct oral anticoagulants are non-inferior to traditional vitamin K antagonists in preventing stroke and arterial thromboembolism in patients with NVAF. The present study reports data from a 1-year follow up in a clinical setting switched from treatment with a VKA to a DOAC for monitoring anticoagulant therapy.12
Materials and Methods
- 196 patients with NVAF with median age of 78.5 years who switched from a vitamin K antagonist to DOAC were prospectively observed after switching from a vitamin K antagonist to dabigatran or rivaroxaban.
- The efficacy, safety and tolerability of the novel treatment, and adherence to it, were evaluated over a period of 1 year.
Results
- A total of 178 patients completed the 1-year follow up, of whom 87 were given dabigatran and 91 rivaroxaban.
- The efficacy of the two DOAC was similar.
- Patients given dabigatran had a higher frequency (n=32) of non-haemorrhagic complications (OR: 3.3; 95% CI: 1.7-7.8), which occurred earlier (HR: 6.1; 95% CI: 3.0-12.6) than those (n=7) recorded in patients on rivaroxaban.
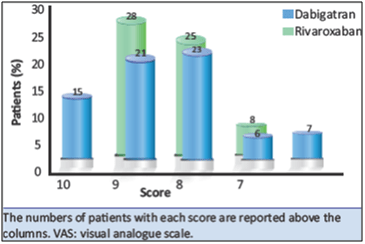
- The degree of satisfaction with rivaroxaban therapy was higher (mean score 9.1, SD 1.0) compared to dabigatran (mean score 8.7; SD 0.9; p=0.01 see Fig. 5).
Conclusion
Direct oral anticoagulants were shown to be effective, safe alternatives to vitamin K antagonists. Additionally, rivaroxaban compared to dabigatran resulted in a lower rate and late occurrence of non-haemorrhagic events, and a higher satisfaction score.
B. Treatment of VTE and Prevention of Recurrent DVT and PE
The EINSTEIN Clinical Development Programme
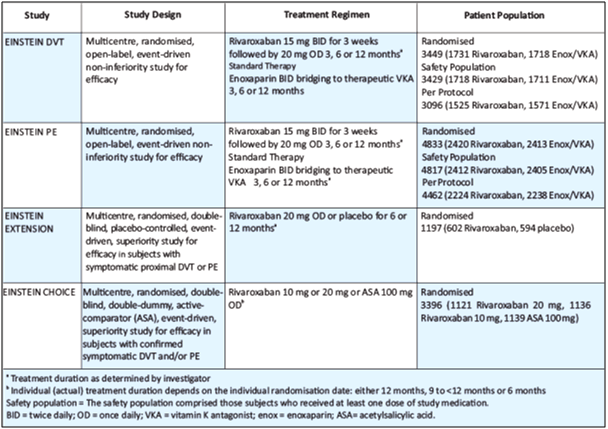
The EINSTEIN clinical development program consisted of four Phase III studies (see Table 4).
- The EINSTEIN DVT and EINSTEIN PE studies evaluated the treatment of VTE and prevention of recurrent DVT and PE.13,14
- The EINSTEIN Extension study evaluated the benefit of continued treatment in patients for whom clinical uncertainty regarding the absolute risk-benefit of extended duration existed. Patients with VTE who were treated either with rivaroxaban or enoxaparin/VKA for 6 or 12 months in EINSTEIN DVT or EINSTEIN PE, or who were treated for 6 to 14 months with VKA and in whom there was equipoise to continue anticoagulant treatment were eligible for enrollment into EINSTEIN Extension.13
- In EINSTEIN CHOICE, patients with confirmed symptomatic VTE who completed 6-12 months of anticoagulant treatment and in whom there was equipoise to continue anticoagulant treatment were eligible for the study. Patients with an indication for continued therapeutic-dosed anticoagulation were excluded.15
- Table 5 depicts the efficacy outcomes in EINSTEIN DVT, EINSTEIN PE and EINSTEIN Extension – ITT population.
EINSTEIN DVT: Clinical Findings13
The primary efficacy outcome for EINSTEIN DVT was recurrent VTE. The principal safety outcome was major bleeding or clinically relevant non major bleeding.
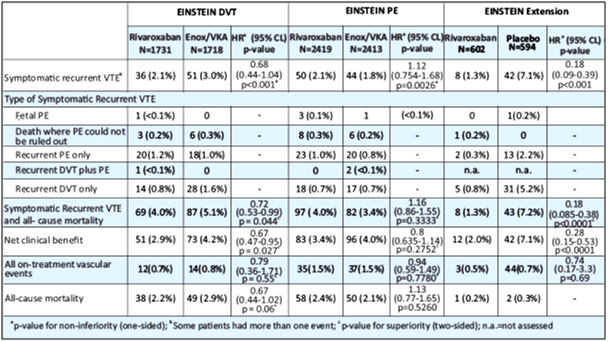
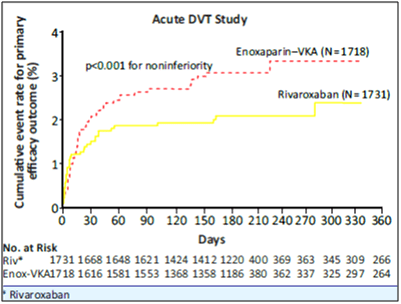
- Rivaroxaban was non-inferior to enoxaparin/VKA for the primary outcome of symptomatic recurrent VTE, 2.1% vs. 3%, respectively (HR of 0.68 [95% CI = 0.44-1.04], p<0.001) (see Table 5 and Fig. 6).
- The results of per-protocol analyses were similar to those of the intention-to-treat analysis. The pre-specified test for superiority was not statistically significant (p= 0.0764).
- The incidence rates for the principal safety outcome (major or clinically relevant non-major bleeding events), as well as the secondary safety outcome (major bleeding events), were similar for both groups (HR of 0.97 [95% CI = 0.76-1.22], p=0.77 and HR of 0.65 [95% CI = 0.33-1.30], p=0.21, respectively).
- The pre-defined secondary outcome of net clinical benefit, (the composite of the primary efficacy outcome and major bleeding events), was reported with a HR of 0.67 ([95% CI = 0.47-0.95], nominal p=0.03) in favour of rivaroxaban. The outcome of a net clinical benefit occurred in 2.9% of patients who received rivaroxaban and 4.2% of patients who received standard therapy (HR, 0.67; 95% CI, 0.47 to 0.95; p=0.03).
- The relative efficacy and safety findings were consistent regardless of pre-treatment (none, LMWH, unfractioned heparin or fondaparinux) as well as among the 3, 6 and 12 month durations. In terms of other secondary outcomes, vascular events during study treatment occurred in 12 patients (0.7%) in the Rivaroxaban arm and 14 patients (0.8%) in the enoxaparin/VKA group (HR of 0.79 [95% CI = 0.36-1.71], p=0.55), and total mortality accounted for 38 (2.2%) vs. 49 (2.9%) patients in the Rivaroxaban vs. enoxaparin/VKA arms, respectively, within intended treatment duration (P = 0.06).
Conclusion
Rivaroxaban offers a simple, single-drug approach to the short-term and continued treatment of venous thrombosis that may improve the benefit-to-risk profile of anticoagulation.
EINSTEIN PE: Clinical Findings14
Rivaroxaban was non-inferior to enoxaparin/VKA for the primary efficacy outcome of symptomatic recurrent VTE, 2.1% vs. 1.8%, respectively (HR of 1.12 [95% CI: 0.75-1.68], p=0.0026) (see Table 5).
- The results of per-protocol analyses were similar to those of the intention-to-treat analysis. The pre-specified test for superiority was not statistically significant (p=0.5737).
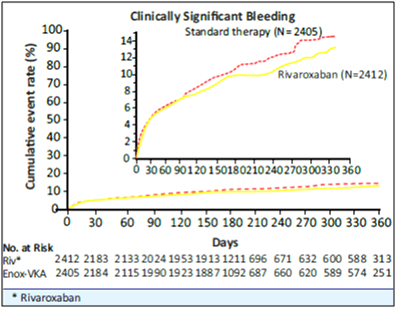
- The incidence rate of the principal safety outcome (major or clinically relevant non-major bleeding events) was similar for both groups (HR of 0.90 [95% CI: 0.76 to 1.07] p=0.2305, see Fig. 7).
- For major bleeding events, the incidence rate was nominally lower in favour of rivaroxaban treatment group (HR of 0.49 [95% CI: 0.31 – 0.79]; p=0.003; see Fig. 8). The pre-defined secondary outcome of net clinical benefit (the composite of the primary efficacy outcome and major bleeding events) was reported with a HR of 0.85 ([95% CI: 0.63-1.14]; P=0.27) in favour of rivaroxaban.
- The relative efficacy and safety findings were consistent regardless of pre-treatment (none, LMWH, unfractioned heparin or fondaparinux) as well as among the 3, 6 and 12 month durations.
Conclusion
Findings of the EINSTEIN PE study involving patients with PE, along with the previous evaluation involving patients with DVT support the use of rivaroxaban as a single oral agent for patients with VTE.
EINSTEIN Extension13
The primary efficacy outcome for EINSTEIN Extension study was recurrent VTE. The principal safety outcome was major bleeding.
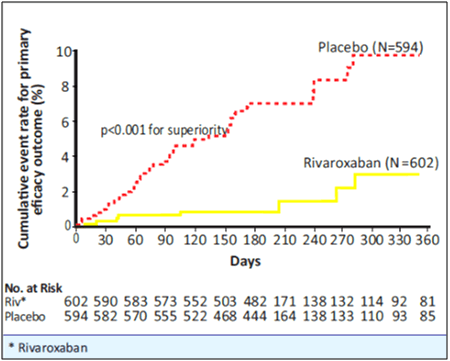
- Rivaroxaban was superior to placebo for the primary efficacy outcome, 1.3% vs. 7.1%, respectively with a HR of 0.18 [95% CI=0.09-0.39], p<0.001 (i.e., a relative risk reduction of 82%) (see Table 5 and Fig. 9).
- The time course for recurrent VTE in rivaroxaban vs. placebo is shown in Figure 9.
- For the principal safety outcome (major bleeding events) there was no significant difference between patients treated with rivaroxaban compared to placebo (p=0.11).
- The pre-defined secondary outcome of net clinical benefit, defined as the composite of the primary efficacy outcome and major bleeding events, was reported with a HR of 0.28 ([95% CI=0.15-0.53], p<0.001) in favour of rivaroxaban.
- In terms of other secondary outcomes, vascular events occurred in 3 patients in the rivaroxaban arm and 4 patients in the placebo group (HR of 0.74 [95% CI=0.17-3.3], p=0.69), and total mortality accounted for 0.2% vs. 0.3% of patients in the rivaroxaban vs. placebo arms, respectively.
Conclusion
Oral rivaroxaban, at a dose of 15 mg BID for the first 3 weeks, followed by 20 mg OD thereafter, without the need for laboratory monitoring provides an effective, safe, single-drug approach to the initial and continued treatment of venous thrombosis.
Einstein Choice15
The primary efficacy outcome was symptomatic recurrent VTE defined as the composite of recurrent DVT or fatal or non-fatal PE. The secondary efficacy outcome was the composite of the primary efficacy outcome, MI, ischemic stroke or non-CNS systemic embolism.
- The primary efficacy outcome for superiority was met for both doses of rivaroxaban 20 mg and 10 mg vs. ASA 100 mg – 1.5% and 1.2% vs. 4.4%, respectively.
- The secondary efficacy outcome was significantly reduced with rivaroxaban 20 mg or 10 mg compared to 100 mg ASA.
- The principal safety outcome (major bleeding events) was similar for patients treated with rivaroxaban 20 mg and 10 mg OD compared to 100 mg ASA.
- The secondary safety outcome (non-major bleeding associated with treatment cessation of more than 14 days) was similar when comparing rivaroxaban 20 mg or 10 mg vs. 100 mg ASA. Outcomes were consistent across the patients with provoked and unprovoked VTE.
- In a prespecified net clinical benefit analysis (NCB) (primary efficacy outcome plus major bleeding events) of EINSTEIN CHOICE, a HR of 0.44 (95% CI 0.27-0.71, p=0.0009) for rivaroxaban 20 mg OD vs. 100 mg ASA OD and a HR of 0.32 (95% CI 0.18 - 0.55, p<0.0001) for rivaroxaban 10 mg OD vs. 100 mg ASA OD were reported.
Conclusion
Rivaroxaban, at both treatment dose (20 mg) and a thromboprophylactic dose (10 mg), was more effective than aspirin for the prevention of recurrent VTE among patients who were in equipoise for continued anticoagulation. The lower risk of a recurrent event among the patients who received rivaroxaban was associated with a rate of bleeding similar to that with aspirin.
C. Rivaroxaban for Prophylaxis of VTE in Acutely Ill Medical Patients
RECORD 1: Superiority of Rivaroxaban on Enoxaparin for Thromboprophylaxis after Total Hip Arthroplasty
Objective
RECORD 1 was a randomized, double-blind, phase 3 trial comparing the efficacy and safety of rivaroxaban to enoxaparin for extended thromboprophylaxis in patients undergoing total hip arthroplasty.16
Design and Methods
- A total of 4541 patients received either 10 mg of oral rivaroxaban OD, beginning after surgery, or 40 mg of enoxaparin subcutaneously (SC) OD, beginning the evening before surgery, plus a placebo tablet or injection.
- The primary efficacy outcome was the composite of DVT (either symptomatic or detected by bilateral venography if the patient was asymptomatic), nonfatal PE, or death from any cause at 36 days (range-30 to 42).
- The main secondary efficacy outcome was major VTE (proximal DVT, nonfatal PE or death from VTE). The primary safety outcome was major bleeding.
Results
- A total of 3153 patients were included in the superiority analysis (after 1388 exclusions) and 4433 were included in the safety analysis (after 108 exclusions).
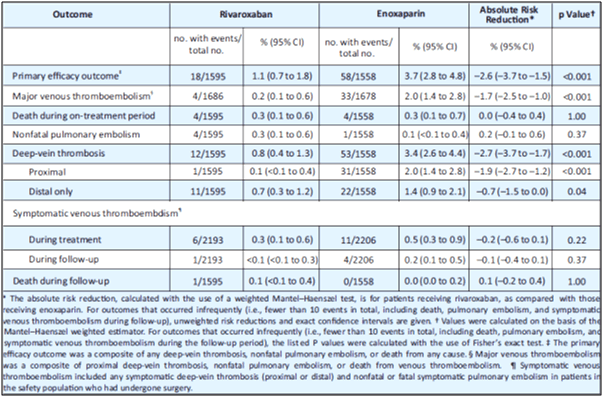
- Rivaroxaban was superior to enoxaparin. The primary efficacy outcome in rivaroxaban group and enoxaparin group was 1.1% vs. 3.7%, respectively (absolute risk reduction, 2.6%; 95% CI, 1.5 to 3.7; p<0.001; see Table 6).
- Rivaroxaban was superior to enoxaparin in terms of occurrence of VTE. Major VTE in the rivaroxaban group vs. enoxaparin group was 0.2% vs. 2.0% (absolute risk reduction, 1.7%; 95% CI, 1.0 to 2.5; p<0.001; see Table 6).
- Major bleeding occurred in 0.3% in the rivaroxaban group and in 0.1% in the enoxaparin group (p=0.18).
Conclusion
A 10 mg oral dose of rivaroxaban OD was significantly more effective for extended thromboprophylaxis than a 40 mg SC dose of enoxaparin in patients undergoing elective THA. Both the drugs showed similar safety profiles.
RECORD 2: Extended Duration Rivaroxaban vs. Short-Term Enoxaparin for the Prevention of VTE after THA
Background and Aim
The risk of VTE is high after THA and could persist after hospital discharge. The present randomized, double-blind, double-dummy, prospective study compared the use of rivaroxaban for extended thromboprophylaxis with short-term thromboprophylaxis with enoxaparin. RECORD 2 study was similar in study design, inclusion/exclusion criteria and endpoints to RECORD 1, except that enoxaparin 40 mg OD (first dose given preoperatively) was given for a shorter duration (12±2 days) than rivaroxaban 10 mg od (35±4 days).
Design and Methods
- A total of 2509 patients scheduled to undergo elective total hip arthroplasty were randomly assigned to receive either oral rivaroxaban 10 mg OD for 31-39 days (with placebo injection for 10-14 days; n=1252) or enoxaparin 40 mg OD SC for 10-14 days (with placebo tablet for 31-39 days; n=1257).
- The primary efficacy outcome was the composite of DVT (symptomatic or asymptomatic detected by mandatory, bilateral venography), non-fatal PE and all-cause mortality up to day 30-42.
- Analyses were done in the modified intention-to-treat population, which consisted of all patients who had received at least one dose of study medication, had undergone planned surgery and had adequate assessment of thromboembolism.
Results
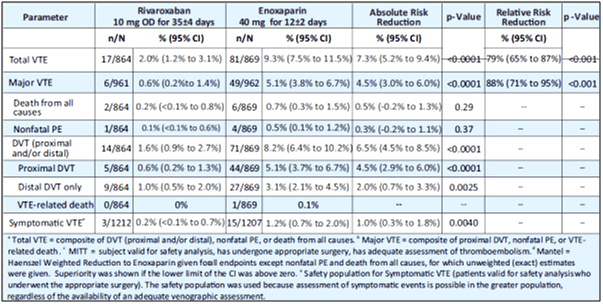
- The modified intention-to-treat population for the analysis of the primary efficacy outcome consisted of 864 patients in the rivaroxaban group and 869 in the enoxaparin group.
- The primary outcome occurred in 2.0% patients in the rivaroxaban group compared with 9.3% in the enoxaparin group (absolute risk reduction 7.3%, 95% CI 5.2-9.4; p<0.0001; see Table 7).
- The incidence of any on-treatment bleeding was much the same in both groups (6.6% events in 1228 patients in the rivaroxaban safety population vs. 5.5% of 1229 patients in the enoxaparin safety population; p=0.25).
Conclusion
Extended thromboprophylaxis with 10 mg rivaroxaban OD for 35 days was significantly more effective with meaningful decrease than short-term enoxaparin plus placebo for the prevention of VTE, including symptomatic events, in patients undergoing total hip arthroplasty.
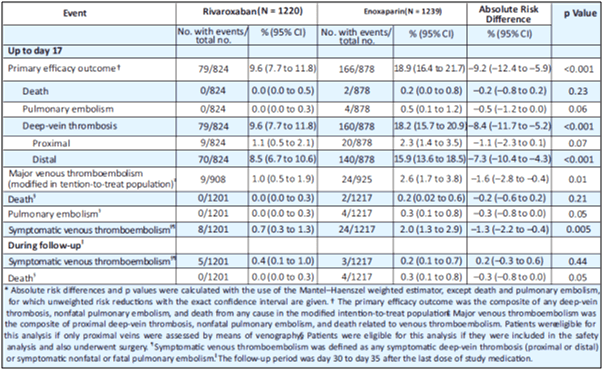
RECORD 3: Superiority of Rivaroxaban over Enoxaparin for Thromboprophylaxis after Total Knee Arthroplasty
Aim
RECORD 3 is a randomized, double-blind trial that investigated the efficacy of rivaroxaban in preventing venous thrombosis after total knee arthroplasty.17
Design and Methods
- A total of 2531 patients who were to undergo total knee arthroplasty received either oral 10 mg rivaroxaban OD, beginning 6 to 8 hours after surgery, or SC 40 mg enoxaparin OD, beginning 12 hours before surgery.
- The primary efficacy outcome was the composite of any DVT, nonfatal PE or death from any cause within 13 to 17 days after surgery.
- Secondary efficacy outcomes included major VTE (i.e., proximal DVT, nonfatal PE or death related to VTE) and symptomatic VTE. The primary safety outcome was major bleeding.
Results
- The primary efficacy outcome occurred in 9.6% who received rivaroxaban and 18.9% who received enoxaparin (absolute risk reduction, 9.2%; 95% CI, 5.9 to 12.4; p<0.001; see Table 8).
- Major VTE occurred in 1% given rivaroxaban and 2.6% given enoxaparin (absolute risk reduction, 1.6%; 95% CI, 0.4 to 2.8; p=0.01; see Table 8).
- Symptomatic events occurred less frequently with rivaroxaban than with enoxaparin (p=0.005).
- Major bleeding occurred in 0.6% of patients in the rivaroxaban group and 0.5% of patients in the enoxaparin group.
- The incidence of drug-related adverse events, mainly gastrointestinal, was 12.0% in the rivaroxaban group and 13.0% in the enoxaparin group.
Conclusion
Rivaroxaban was superior to enoxaparin for thromboprophylaxis after total knee arthroplasty, with similar rates of bleeding.
Post-Discharge Prophylaxis with Rivaroxaban Reduces Fatal and Major Thromboembolic Events in Medically Ill Patients
Background and Aim
Hospitalized acutely ill medical patients are at risk for fatal and major thromboembolic events. The data on the use of extended-duration primary thromboprophylaxis in preventing such events is unknown. Therefore, the study evaluated whether extended-duration rivaroxaban reduces the risk of venous and arterial fatal and major thromboembolic events without significantly increasing major bleeding in acutely ill medical patients after discharge.19
Design and Methods
- Medically ill patients with a baseline creatinine clearance ≥50 ml/min were randomized in a double-blind fashion to rivaroxaban 10 mg or placebo daily at hospital discharge for 45 days.
- In total, 4,909 patients were assigned to rivaroxaban 10 mg and 4,913 patients to placebo. The mean age was 67.8 years, 55.5% were men, mean baseline creatinine clearance was 87.8 ml/min.
- The most frequently reported admitting diagnosis for patients overall was heart failure with reduced ejection fraction ≤45% (37.2%) and the mean duration of hospitalization was 6.7±2.4 days.
- Exploratory efficacy analyses were performed with the intent-to-treat population including all data through day 45. Time-to-event curves were calculated using the Kaplan-Meier method.
Results
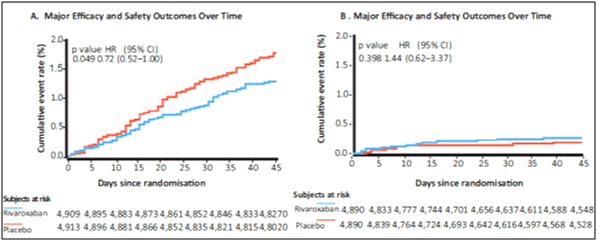
- The pre-specified composite efficacy endpoint (symptomatic VTE, MI, nonhemorrhagic stroke and cardiovascular death) occurred in 1.28% vs. 1.77% of patients in the rivaroxaban vs. placebo groups, respectively (HR: 0.72; 95% CI: 0.52 to 1.00; p=0.049; see Fig. 10A).
- Symptomatic lower-extremity DVT and symptomatic nonfatal PE showed greater risk reduction with rivaroxaban with a relative risk reduction of 80% (HR: 0.20; 95% CI: 0.04 to 0.91) and 62% (HR: 0.36; 95% CI: 0.12 to 1.14), respectively.
- Major bleeding occurred in 0.27% vs. 0.18% of patients in the rivaroxaban vs. placebo groups, respectively (HR: 1.44; 95% CI: 0.62 to 3.37; p=0.398; see Fig. 10B).
- The number of rivaroxaban versus placebo patients was as follows: symptomatic leg DVT (2 vs. 10), symptomatic nonfatal PE (4 vs. 11), MI (13 vs. 8), nonhemorrhagic stroke (13 vs. 24), and cardiovascular death (39 vs. 42).
Conclusions
Extended-duration rivaroxaban at the 10 mg daily dose leads to a significant risk reduction in a composite of fatal and major thromboembolic events without a significant increase in major bleeding compared with placebo. These data suggest that in properly selected patients at risk for VTE and at low risk for bleeding, that extended-duration rivaroxaban at 10 mg has a favorable benefit risk profile.
D. Reduction in Risk of MACE in Patients with Chronic Coronary Artery Disease or Peripheral Artery Disease
COMPASS TRIAL: Stable Atherosclerosis
Background and Objective
Despite the use of effective secondary prevention strategies, 5 to 10% of patients with cardiovascular disease have recurrent events each year. When used for secondary prevention, aspirin results in a 19% lower risk of MACE and a 9% lower risk of cardiovascular death than placebo. Long-term treatment with a vitamin K antagonist, alone or in combination with aspirin, is superior to aspirin for secondary prevention after AMI but is associated with more bleeding, including intracranial bleeding. Thus, anticoagulation has generally not been recommended for patients in this context. The COMPASS trial was a large, international, multicentre randomized controlled trial that evaluated anticoagulation strategies with rivaroxaban among patients with stable atherosclerosis.20
Design and Methods
- The COMPASS trial enrolled 27,395 patients with stable atherosclerotic vascular disease (including CAD and PAD) between 2013 and 2016.
- Patients were randomized (1:1:1) to receive either oral rivaroxaban 2.5 mg BID plus aspirin 100 mg daily (n=9,152), oral rivaroxaban 5 mg BID alone (n=9,117) or 100 mg PO aspirin daily (n=9,126).
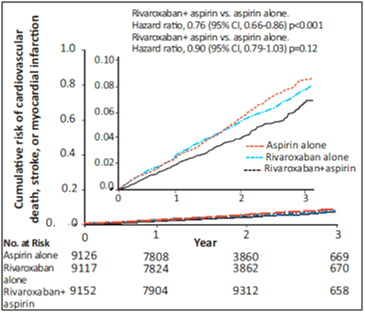
- The primary outcome was a composite of cardiovascular death, stroke or MI. The study was stopped for superiority of the rivaroxaban-plus-aspirin group after a mean follow-up of 23 months.
- Inclusion criteria was Atherosclerosis in ≥2 vascular beds or two additional risk factors (current smoking, diabetes, renal insufficiency, heart failure, or nonlacunar ischemic stroke ≥1 month).
Results
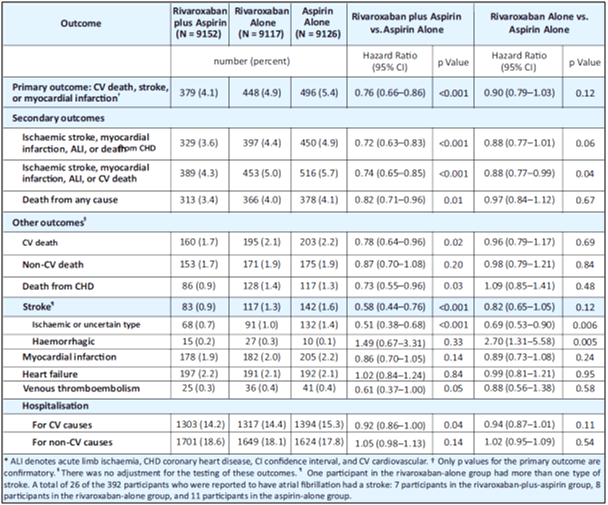
- The primary outcome occurred in fewer patients in the rivaroxaban-plus-aspirin group than in the aspirin-alone group (4.1% vs. 5.4%; HR, 0.76; 95% CI, 0.66 to 0.86; p<0.001; z=−4.126), but major bleeding events occurred in more patients in the rivaroxaban-plus-aspirin group (3.1% vs. 1.9%, HR, 1.70; 95% CI, 1.40 to 2.05; p<0.001). The primary outcome in rivaroxaban alone group was 4.9% (see Fig. 11 and Table 9).
- However, no difference in intracranial or fatal bleeding was observed.
- The rivaroxaban alone arm did not significantly reduce rates of MACE in comparison to aspirin alone, but did increase rates of major bleeding (2.8% vs 1.9%; HR 1.51; 95% CI 1.25–1.84; p < 0.001).
- Death rate in the rivaroxaban-plus-aspirin group vs. aspirin-alone group was 3.4% vs. 4.1%, respectively (hazard ratio, 0.82; 95% CI, 0.71 to 0.96; P=0.01; threshold P value for significance, 0.0025).
- The secondary composite outcome of ischemic stroke, MI, acute limb ischemia or death from CHD occurred in fewer patients in the rivaroxaban-plus-aspirin group than in the aspirin-alone group (3.6% vs. 4.9%; HR, 0.72; 95% CI, 0.63 to 0.83; p<0.001) (see Table 9).
- The secondary outcome of ischemic stroke, MI, acute limb ischemia, or cardiovascular death also occurred in fewer patients in the rivaroxaban-plus-aspirin group than in the aspirin-alone group (4.3% vs. 5.7%; HR, 0.74; 95% CI, 0.65 to 0.85; p<0.001).
Conclusion
The COMPASS trial showed that rivaroxaban plus aspirin was associated with fewer adverse cardiovascular events.
COMPASS-Heart Failure
Background and Objective
Patients with CAD or PAD who also have HF have nearly a 2-fold higher risk of subsequent cardiovascular events than those without HF. Furthermore, these patients are at high risk for MACE. The COMPASS excluded patients with recently decompensated HF or severe HF as defined by baseline EF of ≤30% or New York Heart Association functional class III or IV HF. The present study evaluated the effects of rivaroxaban with or without aspirin on MACE and bleeding in COMPASS patients with or without a history of HF and according to left ventricular EF recorded at baseline.21
Design and Methods
- Of the 27,395 patients enrolled in COMPASS, 5902 (22%) had a history of HF at baseline. Left ventricular EF was available in 16,792 patients (61.3%), including 4971 of 5902 (84.2%) of those with HF.
- The primary major adverse cardiovascular events outcome comprised cardiovascular death, stroke, or MI, and the primary safety outcome was major bleeding using modified International Society of Thrombosis and Haemostasis criteria.
Results
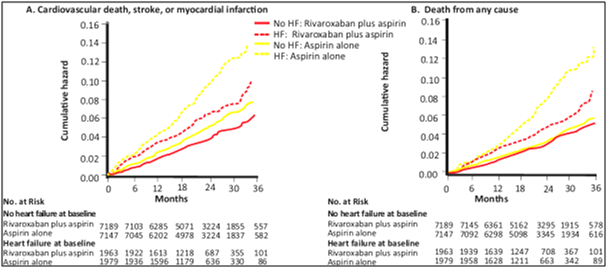
- MACE: Rivaroxaban plus aspirin had similar relative reduction in MACE compared with aspirin in participants with HF (5.5% versus 7.9%; hazard ratio [HR], 0.68; 95% CI, 0.53–0.86) and those without HF (3.8% versus 4.7%; HR, 0.79; 95% CI, 0.68–0.93; P for interaction 0.28) but larger absolute risk reduction in those with HF (HF absolute risk reduction 2.4%, number needed to treat=42; no HF absolute risk reduction 1.0%, number needed to treat=103) (see Fig. 12).
- Hospitalisation for heart failure: Rivaroxaban plus aspirin vs. aspirin alone was associated with greater benefit among those with heart failure vs. those without heart failure (p for interaction = 0.05)
- All-cause mortality: Rivaroxaban plus aspirin vs. aspirin alone was associated with greater benefit among those with heart failure vs. those without heart failure (p for interaction = 0.05)
Conclusion
In patients with chronic CAD or PAD, rivaroxaban 2.5 mg BID plus aspirin as compared to aspirin alone produces similar relative risk reductions but larger absolute risk benefits in patients with mild to moderate HF who do not have recent decompensated HF or advanced HFrEF.
COMPASS-PAD
Among 7,470 participants with PAD, 4,129 had symptomatic PAD, 1,919 had carotid disease and 1,422 had coronary artery disease plus ankle-brachial index <0.9.22
- MACE: MACE occurred in fewer patients in the rivaroxaban-plus-aspirin group than the rivaroxaban alone and the aspirin-alone group 5.0% vs. 6.0% vs. 7.0%, respectively (p=0.005 for rivaroxaban plus aspirin vs. aspirin alone; p=0.19 for rivaroxaban alone vs. aspirin alone).
- Major adverse limb events (MALE): MALE occurred in fewer patients in the rivaroxaban-plus-aspirin group than the rivaroxaban alone and the aspirin-alone group 1.5% vs. 1.9% vs. 2.6%, respectively (p=0.01 for rivaroxaban plus aspirin vs. aspirin alone; p=0.07 for rivaroxaban alone vs. aspirin alone).
- Major bleeding: 3.0% for rivaroxaban plus aspirin, 3.0% for rivaroxaban alone, vs. 2.0% for aspirin alone (p=0.009 for rivaroxaban plus aspirin vs. aspirin alone; p=0.004 for rivaroxaban alone vs. aspirin alone).
COMPASS-CAD
There were 24,824 subjects with coronary artery disease (CAD).22
- MACE: MACE occurred in fewer patients in the rivaroxaban-plus-aspirin group than the rivaroxaban alone and the aspirin-alone group 4.0% vs. 5.0% vs. 6.0%, respectively (p < 0.0001 for rivaroxaban plus aspirin vs. aspirin alone; p = 0.094 for rivaroxaban alone vs. aspirin alone).
- Major bleeding: 3.0% for rivaroxaban plus aspirin, 3.0% for rivaroxaban alone, vs. 2.0% for aspirin alone (p<0.0001 for rivaroxaban plus aspirin vs. aspirin alone; p<0.0001 for rivaroxaban alone vs. aspirin alone).
COMPASS-Vascular Risk
In this analysis, patients were classified as high-risk according to REACH scoring (2 or more vascular beds affected, history of heart failure, or renal insufficiency) or CART scoring (2 or more vascular beds affected, history of heart failure, or diabetes).22
- High-risk features-REACH, MACE, acute limb ischemia, or total vascular amputation: 5.8% with rivaroxaban plus aspirin vs. 8.0% with aspirin (events prevented per 1,000 patients treated [95% CI]=36 [21-52]).
- High-risk features-CART, MACE, acute limb ischemia, or total vascular amputation: 5.6% with rivaroxaban plus aspirin vs. 7.5% with aspirin (events prevented per 1,000 patients treated [95% CI]=33 [19-47]).
- High-risk features-REACH, severe bleeding: 1.1% with rivaroxaban plus aspirin vs. 0.8% with aspirin (events caused per 1,000 patients treated [95% CI]=3 [-4 to 9]).
- High-risk features-CART, severe bleeding: 1.0% with rivaroxaban plus aspirin versus 0.8% with aspirin (events caused per 1,000 patients treated [95% CI]=1 [-4 to 6]).
Among patients with stable atherosclerosis, rivaroxaban plus aspirin was associated with fewer adverse cardiovascular events. Rivaroxaban plus aspirin compared with aspirin alone was associated with a reduction in all strokes and ischemic strokes. Rivaroxaban alone compared with aspirin alone was not associated with a reduction in all strokes. Rivaroxaban plus aspirin was effective at preventing both MACE and MALE among those with PAD. Patients with chronic CAD or PAD given a combination of rivaroxaban and ASA had significantly better prognosis when compared to patients receiving aspirin alone.
Prescribing Information
|
Warning: (A) Premature Discontinuation of RIVAZEST Increases the Risk of Thrombotic Events (B) Spinal/Epidural Hematoma A. Premature discontinuation of RIVAZEST increases the risk of thrombotic events- Premature discontinuation of any oral anticoagulant, including RIVAZEST, increases the risk of thrombotic events. If anticoagulation with RIVAZEST is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant. B. Spinal/epidural hematoma- Epidural or spinal hematomas have occurred in patients treated with RIVAZEST who are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
Monitor patients frequently for signs and symptoms of neurological impairment. If neurological compromise is noted, urgent treatment is necessary. Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated for thromboprophylaxis. |
RIVAZEST (Rivaroxaban) Tablets
Generic Name: Rivaroxaban
Quantitative and Qualitative Composition
Each film-coated tablet contains 2.5 mg rivaroxaban
Each film-coated tablet contains 10 mg rivaroxaban
Each film-coated tablet contains 15 mg rivaroxaban
Each film-coated tablet contains 20 mg rivaroxaban
Dosage Form & Strength
2.5 mg tablets, 10 mg tablets, 15 mg tablets, 20 mg tablets
Therapeutic Indication
- Reduction of Risk of Stroke and Systemic Embolism in Nonvalvular Atrial Fibrillation
- Treatment of Deep Vein Thrombosis
- Treatment of Pulmonary Embolism
- Reduction in the Risk of Recurrence of Deep Vein Thrombosis and/or Pulmonary Embolism
- Prophylaxis of Deep Vein Thrombosis Following Hip or Knee Replacement Surgery
- Reduction of Risk of Major Cardiovascular Events in Patients with Chronic Coronary Artery Disease (CAD) or Peripheral Artery Disease (PAD)
Dosage and Administration
A. Recommended Dosage
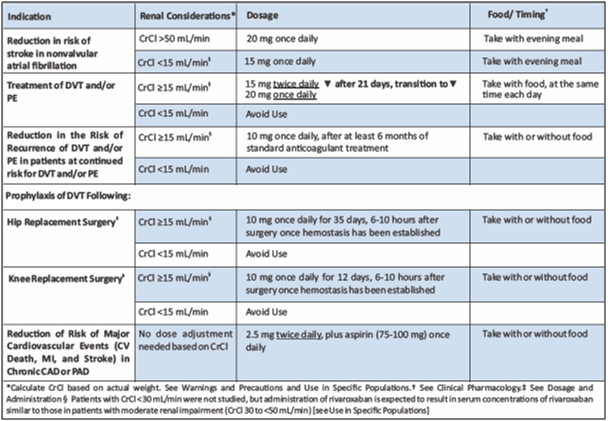
Table 10 depicts dosage and administration of Rivaroxaban.
B. Switching to and from Rivaroxaban
1. Switching from Warfarin to Rivaroxaban - When switching patients from warfarin to rivaroxaban, discontinue warfarin and start rivaroxaban as soon as the International Normalized Ratio (INR) is below 3.0 to avoid periods of inadequate anticoagulation.
2. Switching from Rivaroxaban to Warfarin - No clinical trial data are available to guide converting patients from rivaroxaban to warfarin. Rivaroxaban affects INR, so INR measurements made during coadministration with warfarin may not be useful for determining the appropriate dose of warfarin. One approach is to discontinue rivaroxaban and begin both a parenteral anticoagulant and warfarin at the time the next dose of rivaroxaban would have been taken.
3. Switching from Rivaroxaban to Anticoagulants other than Warfarin - For patients currently taking rivaroxaban and transitioning to an anticoagulant with rapid onset, discontinue rivaroxaban and give the first dose of the other anticoagulant (oral or parenteral) at the time that the next rivaroxaban dose would have been taken.
4. Switching from Anticoagulants other than Warfarin to Rivaroxaban - For patients currently receiving an anticoagulant other than warfarin, start rivaroxaban 0 to 2 hours prior to the next scheduled evening administration of the drug (e.g., low molecular weight heparin or non-warfarin oral anticoagulant) and omit administration of the other anticoagulant. For unfractionated heparin being administered by continuous infusion, stop the infusion and start rivaroxaban at the same time.
C. Discontinuation for Surgery and other Interventions
If anticoagulation must be discontinued to reduce the risk of bleeding with surgical or other procedures, rivaroxaban should be stopped at least 24 hours before the procedure to reduce the risk of bleeding. In deciding whether a procedure should be delaye until 24 hours after the last dose of rivaroxaban, the increased risk of bleeding should be weighed against the urgency of intervention. Rivaroxaban should be restarted after the surgical or other procedures as soon as adequate hemostasis has been established, noting that the time to onset of therapeutic effect is short [see Warnings and Precautions]. If oral medication cannot be taken during or after surgical intervention, consider administering a parenteral anticoagulant.
D. Missed Dose
- For patients receiving 2.5 mg twice daily: if a dose is missed, the patient should take a single 2.5 mg rivaroxaban dose as recommended at the next scheduled time.
- For patients receiving 15 mg twice daily: The patient should take rivaroxaban immediately to ensure intake of 30 mg rivaroxaban per day. Two 15 mg tablets may be taken at once.
- For patients receiving 20 mg, 15 mg or 10 mg once daily: The patient should take the missed rivaroxaban dose immediately. The dose should not be doubled within the same day to make up for a missed dose.
Contraindication
Rivaroxaban is contraindicated in patients with active pathological bleeding & severe hypersensitivity reaction to rivaroxaban (e.g., anaphylactic reactions)
Warnings & Precautions
Increased Risk of Thrombotic Events after Premature Discontinuation
Premature discontinuation of any oral anticoagulant, including rivaroxaban, in the absence of adequate alternative anticoagulation increases the risk of thrombotic events. An increased rate of stroke was observed during the transition from rivaroxaban to warfarin in clinical trials in atrial fibrillation patients. If rivaroxaban is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant.
Risk of Bleeding
Rivaroxaban increases the risk of bleeding and can cause serious or fatal bleeding. In deciding whether to prescribe rivaroxaban to patients at increased risk of bleeding, the risk of thrombotic events should be weighed against the risk of bleeding. Promptly evaluate any signs or symptoms of blood loss and consider the need for blood replacement. Discontinue rivaroxaban in patients with active pathological hemorrhage. The terminal elimination half-life of rivaroxaban is 5 to 9 hours in healthy subjects aged 20 to 45 years. Concomitant use of other drugs that impair hemostasis increases the risk of bleeding. These include aspirin, P2Y12 platelet inhibitors, dual antiplatelet therapy, other antithrombotic agents, fibrinolytic therapy, non-steroidal anti- inflammatory drugs (NSAIDs), selective serotonin reuptake inhibitors, and serotonin norepinephrine reuptake inhibitors. Concomitant use of drugs that are known combined P-gp and strong CYP3A inhibitors increases rivaroxaban exposure and may increase bleeding risk.
A. Risk of Hemorrhage in Acutely Ill Medical Patients at High Risk of Bleeding
Acutely ill medical patients with the following conditions are at increased risk of bleeding with the use of rivaroxaban for primary VTE prophylaxis: history of bronchiectasis, pulmonary cavitation, or pulmonary hemorrhage, active cancer (i.e. undergoing acute, in-hospital cancer treatment), active gastroduodenal ulcer in the three months prior to treatment, history of bleeding in the three months prior to treatment, or dual antiplatelet therapy. Rivaroxaban is not for use for primary VTE prophylaxis in these hospitalized, acutely ill medical patients at high risk of bleeding.
B. Reversal of Anticoagulant Effect
An agent to reverse the anti-factor Xa activity of rivaroxaban is available. Because of high plasma protein binding, rivaroxaban is not dialyzable [see Clinical Pharmacology]. Protamine sulfate and vitamin K are not expected to affect the anticoagulant activity of rivaroxaban. Use of procoagulant reversal agents, such as prothrombin complex concentrate (PCC), activated prothrombin complex concentrate or recombinant factor VIIa, may be considered but has not been evaluated in clinical efficacy and safety studies. Monitoring for the anticoagulation effect of rivaroxaban using a clotting test (PT, INR or aPTT) or anti-factor Xa (FXa) activity is not recommended.
C. Spinal/Epidural Anesthesia or Puncture
When neuraxial anesthesia (spinal/epidural anesthesia) or spinal puncture is employed, patients treated with anticoagulant agents for prevention of thromboembolic complications are at risk of developing an epidural or spinal hematoma which can result in long-term or permanent paralysis [see Boxed Warning].To reduce the potential risk of bleeding associated with the concurrent use of rivaroxaban and epidural or spinal anesthesia/analgesia or spinal puncture, consider the pharmacokinetic profile of rivaroxaban. Placement or removal of an epidural catheter or lumbar puncture is best performed when the anticoagulant effect of rivaroxaban is low; however, the exact timing to reach a sufficiently low anticoagulant effect in each patient is not known. An indwelling epidural or intrathecal catheter should not be removed before at least 2 half-lives have elapsed (i.e., 18 hours in young patients aged 20 to 45 years and 26 hours in elderly patients aged 60 to 76 years), after the last administration of rivaroxaban [see Clinical Pharmacology]. The next rivaroxaban dose should not be administered earlier than 6 hours after the removal of the catheter. If traumatic puncture occurs, delay the administration of rivaroxaban for 24 hours. Should the physician decide to administer anticoagulation in the context of epidural or spinal anesthesia/analgesia or lumbar puncture, monitor frequently to detect any signs or symptoms of neurological impairment, such as midline back pain, sensory and motor deficits (numbness, tingling, or weakness in lower limbs), bowel and/or bladder dysfunction. Instruct patients to immediately report if they experience any of the above signs or symptoms. If signs or symptoms of spinal hematoma are suspected, initiate urgent diagnosis and treatment including consideration for spinal cord decompression even though such treatment may not prevent or reverse neurological sequelae.
D. Use in Patients with Renal Impairment
1. Nonvalvular Atrial Fibrillation- Periodically assess renal function as clinically indicated (i.e., more frequently in situations in which renal function may decline) and adjust therapy accordingly. Consider dose adjustment or discontinuation of rivaroxaban in patients who develop acute renal failure while on rivaroxaban.
2. Treatment of Deep Vein Thrombosis (DVT), Pulmonary Embolism (PE), and Reduction in the Risk of Recurrence of DVT and of PE- In patients with CrCl <30 mL/min, rivaroxaban exposure and pharmacodynamic effects are increased compared to patients with normal renal function. There are limited clinical data in patients with CrCl 15 to <30 mL/min; therefore, observe closely and promptly evaluate any signs or symptoms of blood loss in these patients. There are no clinical data in patients with CrCl <15 mL/min (including patients on dialysis); therefore, avoid the use of rivaroxaban in these patients. Discontinue rivaroxaban in patients who develop acute renal failure while on treatment.
3. Prophylaxis of Deep Vein Thrombosis Following Hip or Knee Replacement Surgery- In patients with CrCl <30 mL/min, rivaroxaban exposure and pharmacodynamic effects are increased compared to patients with normal renal function. There are limited clinical data in patients with CrCl 15 to <30 mL/min; therefore, observe closely and promptly evaluate any signs or symptoms of blood loss in these patients. There are no clinical data in patients with CrCl <15 mL/min (including patients on dialysis); therefore, avoid the use of rivaroxaban in these patients. Discontinue rivaroxaban in patients who develop acute renal failure while on treatment.
4. Prophylaxis of Venous Thromboembolism in Acutely Ill Medical Patients at Risk for Thromboembolic Complications Not at High Risk of Bleeding In patients with CrCl <30 mL/min, rivaroxaban exposure and pharmacodynamic effects are increased compared to patients with normal renal function. There are limited clinical data in patients with CrCl 15 to <30 mL/min; therefore, observe closely and promptly evaluate any signs or symptoms of blood loss in these patients. There are no clinical data in patients with CrCl <15 mL/min (including patients on dialysis); therefore, avoid the use of rivaroxaban in these patients. Discontinue rivaroxaban in patients who develop acute renal failure while on treatment.
E. Use in Patients with Hepatic Impairment
No clinical data are available for patients with severe hepatic impairment. Avoid use of rivaroxaban in patients with moderate (Child-Pugh B) and severe (Child-Pugh C) hepatic impairment or with any hepatic disease associated with coagulopathy since drug exposure and bleeding risk may be increased.
F. Use with P-gp and Strong CYP3A Inhibitors or Inducers
Avoid concomitant use of rivaroxaban with known combined P-gp and strong CYP3A inhibitors. Avoid concomitant use of rivaroxaban with drugs that are known combined P-gp and strong CYP3A inducers.
G. Risk of Pregnancy-Related Hemorrhage
In pregnant women, rivaroxaban should be used only if the potential benefit justifies the potential risk to the mother and fetus. Rivaroxaban dosing in pregnancy has not been studied. The anticoagulant effect of rivaroxaban cannot be monitored with standard laboratory testing. Promptly evaluate any signs or symptoms suggesting blood loss (e.g., a drop in hemoglobin and/or hematocrit, hypotension, or fetal distress).
H. Patients with Prosthetic Heart Valves
On the basis of the GALILEO study, use of rivaroxaban is not recommended in patients who have had transcatheter aortic valve replacement (TAVR) because patients randomized to rivaroxaban experienced higher rates of death and bleeding compared to those randomized to an anti-platelet regimen. The safety and efficacy of rivaroxaban have not been studied in patients with other prosthetic heart valves or other valve procedures. Use of rivaroxaban is not recommended in patients with prosthetic heart valves.
I. Acute PE in Hemodynamically Unstable Patients or Patients Who Require Thrombolysis or Pulmonary Embolectomy
Initiation of rivaroxaban is not recommended acutely as an alternative to unfractionated heparin in patients with pulmonary embolism who present with hemodynamic instability or who may receive thrombolysis or pulmonary embolectomy.
J. Increased Risk of Thrombosis in Patients with Triple Positive Antiphospholipid Syndrome
Direct-acting oral anticoagulants (DOACs), including rivaroxaban, are not recommended for use in patients with triple-positive antiphospholipid syndrome (APS). For patients with APS (especially those who are triple positive [positive for lupus anticoagulant, anticardiolipin, and anti-beta 2-glycoprotein I antibodies]), treatment with DOACs has been associated with increased rates of recurrent thrombotic events compared with vitamin K antagonist therapy.
Use in Specific Populations
A. Pregnancy
1. Risk Summary
The limited available data on rivaroxaban in pregnant women are insufficient to inform a drug-associated risk of adverse developmental outcomes. Use rivaroxaban with caution in pregnant patients because of the potential for pregnancy related hemorrhage and/or emergent delivery. The anticoagulant effect of rivaroxaban cannot be reliably monitored with standard laboratory testing. Consider the benefits and risks of rivaroxaban for the mother and possible risks to the fetus when prescribing rivaroxaban to a pregnant woman.
Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
2. Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Pregnancy is a risk factor for venous thromboembolism and that risk is increased in women with inherited or acquired thrombophilias. Pregnant women with thromboembolic disease have an increased risk of maternal complications including pre-eclampsia. Maternal thromboembolic disease increases the risk for intrauterine growth restriction, placental abruption and early and late pregnancy loss.
Fetal/Neonatal adverse Reactions
Based on the pharmacologic activity of Factor Xa inhibitors and the potential to cross the placenta, bleeding may occur at any site in the fetus and/or neonate.
Labor or delivery
All patients receiving anticoagulants, including pregnant women, are at risk for bleeding and this risk may be increased during labor or delivery [see Warnings and Precautions (5.7)]. The risk of bleeding should be balanced with the risk of thrombotic events when considering the use of rivaroxaban in this setting.
3. Data
Human Data
There are no adequate or well-controlled studies of rivaroxaban in pregnant women, and dosing for pregnant women has not been established. Post-marketing experience is currently insufficient to determine a rivaroxaban-associated risk for major birth defects or miscarriage. In an in vitro placenta perfusion model, unbound rivaroxaban was rapidly transferred across the human placenta.
B. Lactation
Risk Summary
Rivaroxaban has been detected in human milk. There are insufficient data to determine the effects of rivaroxaban on the breastfed child or on milk production. Rivaroxaban and/or its metabolites were present in the milk of rats. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for rivaroxaban and any potential adverse effects on the breastfed infant from rivaroxaban or from the underlying maternal condition.
C. Females and Males of Reproductive Potential
Females of reproductive potential requiring anticoagulation should discuss pregnancy planning with their physician
D. Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
E. Geriatric Use
Of the total number of patients in the RECORD 1-3 clinical studies evaluating rivaroxaban, about 54% were 65 years and over, while about 15% were >75 years. In ROCKET AF, approximately 77% were 65 years and over and about 38% were >75 years. In the EINSTEIN DVT, PE and Extension clinical studies approximately 37% were 65 years and over and about 16% were >75 years. In EINSTEIN CHOICE, approximately 39% were 65 years and over and about 12% were >75 years. In the MAGELLAN study, approximately 67% were 65 years and over and about 37% were >75 years. In the COMPASS study, approximately 76% were 65 years and over and about 17% were >75 years. In clinical trials the efficacy of rivaroxaban in the elderly (65 years or older) was similar to that seen in patients younger than 65 years. Both thrombotic and bleeding event rates were higher in these older patients.
F. Renal Impairment
In pharmacokinetic studies, compared to healthy subjects with normal creatinine clearance, rivaroxaban exposure increased by approximately 44 to 64% in subjects with renal impairment. Increases in pharmacodynamic effects were also observed.
Undesirable Effects
The following clinically significant adverse reactions are also discussed in other sections of the labeling:
- Increased Risk of Stroke After Discontinuation in Nonvalvular Atrial Fibrillation [see Boxed Warning and Warnings and Precautions]
- Bleeding Risk [see Warnings and Precautions]
- Spinal/Epidural Hematoma
Overdosage
Overdose of rivaroxaban may lead to hemorrhage. Discontinue rivaroxaban and initiate appropriate therapy if bleeding complications associated with overdosage occur. Rivaroxaban systemic exposure is not further increased at single doses >50 mg due to limited absorption. The use of activated charcoal to reduce absorption in case of rivaroxaban overdose may be considered. Due to the high plasma protein binding, rivaroxaban is not dialyzable [see Warnings and Precautions and Clinical Pharmacology]. Partial reversal of laboratory anticoagulation parameters may be achieved with use of plasma products. An agent to reverse the anti-factor Xa activity of rivaroxaban is available.
Clinical Pharmacology
A. Mechanism of Action- Rivaroxaban is a selective inhibitor of FXa. It does not require a cofactor (such as Anti-thrombin III) for activity. Rivaroxaban inhibits free FXa and prothrombinase activity. Rivaroxaban has no direct effect on platelet aggregation, but indirectly inhibits platelet aggregation induced by thrombin. By inhibiting FXa, rivaroxaban decreases thrombin generation.
B. Pharmacodynamics- Dose-dependent inhibition of FXa activity was observed in humans. Neoplastin® prothrombin time (PT), activated partial thromboplastin time (aPTT) and HepTest® are also prolonged dose-dependently. Anti-factor Xa activity is also influenced by rivaroxaban.
Specific Populations
1. Renal Impairment- The relationship between systemic exposure and pharmacodynamic activity of rivaroxaban was altered in subjects with renal impairment relative to healthy control subjects.
2. Hepatic Impairment- Anti-Factor Xa activity was similar in subjects with normal hepatic function and in mild hepatic impairment (Child-Pugh A class). There is no clear understanding of the impact of hepatic impairment beyond this degree on the coagulation cascade and its relationship to efficacy and safety.
C. Pharmacokinetics
1. Absorption
The absolute bioavailability of rivaroxaban is dose-dependent. For the 2.5 mg and 10 mg dose, it is estimated to be 80% to 100% and is not affected by food. Rivaroxaban 2.5 mg and 10 mg tablets can be taken with or without food. For the 20 mg dose in the fasted state, the absolute bioavailability is approximately 66%. Coadministration of rivaroxaban with food increases the bioavailability of the 20 mg dose (mean AUC and Cmax increasing by 39% and 76% respectively with food). Rivaroxaban 15 mg and 20 mg tablets should be taken with food.
The maximum concentrations (Cmax) of rivaroxaban appear 2 to 4 hours after tablet intake. The pharmacokinetics of rivaroxaban were not affected by drugs altering gastric pH. Coadministration of rivaroxaban (30 mg single dose) with the H2-receptor antagonist ranitidine (150 mg twice daily), the antacid aluminum hydroxide/magnesium hydroxide (10 mL) or rivaroxaban (20 mg single dose) with the PPI omeprazole (40 mg once daily) did not show an effect on the bioavailability and exposure of rivaroxaban (see Figure 2). Absorption of rivaroxaban is dependent on the site of drug release in the GI tract. Avoid administration of rivaroxaban distal to the stomach which can result in reduced absorption and related drug exposure.
2. Distribution
Plasma protein binding of rivaroxaban in human plasma is approximately 92% to 95%, with albumin being the main binding component. The steady-state volume of distribution in healthy subjects is approximately 50 L.
3. Metabolism
Approximately 51% of an orally administered [14C]-rivaroxaban dose was recovered as inactive metabolites in urine (30%) and feces (21%). Oxidative degradation catalyzed by CYP3A4/5 and CYP2J2 and hydrolysis are the major sites of biotransformation.
4. Excretion
In a Phase 1 study, following the administration of [14C]-rivaroxaban, approximately one- third (36%) was recovered as unchanged drug in the urine and 7% was recovered as unchanged drug in feces. Unchanged drug is excreted into urine, mainly via active tubular secretion and to a lesser extent via glomerular filtration (approximate 5:1 ratio). Rivaroxaban is a substrate of the efflux transporter proteins P-gp and ABCG2 (also abbreviated Bcrp). Rivaroxaban’s affinity for influx transporter proteins is unknown. Rivaroxaban is a low-clearance drug, with a systemic clearance of approximately 10 L/hr in healthy volunteers following intravenous administration. The terminal elimination half-life of rivaroxaban is 5 to 9 hours in healthy subjects aged 20 to 45 years.
D. Specific Populations
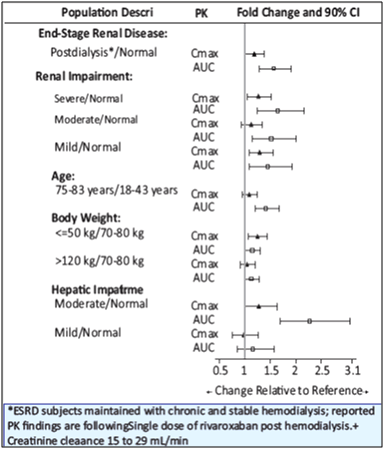
The effects of level of renal impairment, age, body weight, and level of hepatic impairment on the pharmacokinetics of rivaroxaban are summarized in Figure 13.
1. Gender- Gender did not influence the pharmacokinetics or pharmacodynamics of rivaroxaban.
2. Race- Healthy Japanese subjects were found to have 20 to 40% on average higher exposures compared to other ethnicities including Chinese. However, these differences in exposure are reduced when values are corrected for body weight.
3. Elderly- The terminal elimination half-life is 11 to 13 hours in the elderly subjects aged 60 to 76 years.
4. Renal Impairment- The safety and pharmacokinetics of single-dose rivaroxaban (10 mg) were evaluated in a study in healthy subjects [CrCl ≥80 mL/min (n=8)] and in subjects with varying degrees of renal impairment (see Figure 1). Compared to healthy subjects with normal creatinine clearance, rivaroxaban exposure increased in subjects with renal impairment. Increases in pharmacodynamic effects were also observed.
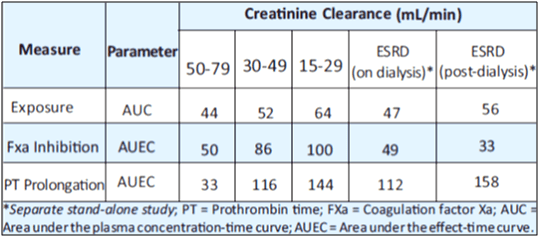
5. Hemodialysis in ESRD subjects: Systemic exposure to rivaroxaban administered as a single 15 mg dose in ESRD subjects dosed 3 hours after the completion of a 4-hour hemodialysis session (post-dialysis) is 56% higher when compared to subjects with normal renal function (see Table 2). The systemic exposure to rivaroxaban administered 2 hours prior to a 4-hour hemodialysis session with a dialysate flow rate of 600 mL/min and a blood flow rate in the range of 320 to 400 mL/min is 47% higher compared to those with normal renal function. The extent of the increase is similar to the increase in patients with CrCl 15 to 50 mL/min taking rivaroxaban 15 mg.
6. Hepatic Impairment- The safety and pharmacokinetics of single-dose rivaroxaban (10 mg) were evaluated in a study in healthy subjects (n=16) and subjects with varying degrees of hepatic impairment (see Figure 1). No patients with severe hepatic impairment (Child-Pugh C) were studied. Compared to healthy subjects with normal liver function, significant increases in rivaroxaban exposure were observed in subjects with moderate hepatic impairment (Child-Pugh B) (see Figure 1). Increases in pharmacodynamic effects were also observed.
Drug Interactions
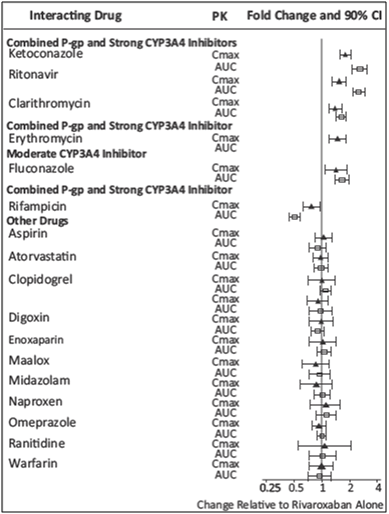
In vitro studies indicate that rivaroxaban neither inhibits the major cytochrome P450 enzymes CYP1A2, 2C8, 2C9, 2C19, 2D6, 2J2, and 3A nor induces CYP1A2, 2B6, 2C19, or 3A. In vitro data also indicates a low rivaroxaban inhibitory potential for P-gp and ABCG2 transporters. The effects of coadministered drugs on the pharmacokinetics of rivaroxaban exposure are summarized in Figure 14 [see Drug Interactions].
1. Anticoagulants
In a drug interaction study, single doses of enoxaparin (40 mg subcutaneous) and rivaroxaban (10 mg) given concomitantly resulted in an additive effect on anti-factor Xa activity. In another study, single doses of warfarin (15 mg) and rivaroxaban (5 mg) resulted in an additive effect on factor Xa inhibition and PT. Neither enoxaparin nor warfarin affected the pharmacokinetics of rivaroxaban (see Figure 2).
2. NSAIDs/Aspirin
In ROCKET AF, concomitant aspirin use (almost exclusively at a dose of 100 mg or less) during the double-blind phase was identified as an independent risk factor for major bleeding. NSAIDs are known to increase bleeding, and bleeding risk may be increased when NSAIDs are used concomitantly with rivaroxaban. Neither naproxen nor aspirin affected the pharmacokinetics of rivaroxaban (see Figure 2).
3. Clopidogrel
In two drug interaction studies where clopidogrel (300 mg loading dose followed by 75 mg daily maintenance dose) and rivaroxaban (15 mg single dose) were coadministered in healthy subjects, an increase in bleeding time to 45 minutes was observed in approximately 45% and 30% of subjects in these studies, respectively. The change in bleeding time was approximately twice the maximum increase seen with either drug alone. There was no change in the pharmacokinetics of either drug.
4. Drug-Disease Interactions with Drugs that Inhibit Cytochrome P450 3A Enzymes and Drug Transport Systems
In a pharmacokinetic trial, rivaroxaban was administered as a single dose in subjects with mild (CrCl = 50 to 79 mL/min) or moderate renal impairment (CrCl = 30 to 49 mL/min) receiving multiple doses of erythromycin (a combined P-gp and moderate CYP3A inhibitor). Compared to rivaroxaban administered alone in subjects with normal renal function (CrCl >80 mL/min), subjects with mild and moderate renal impairment concomitantly receiving erythromycin reported a 76% and 99% increase in AUCinf and a 56% and 64% increase in Cmax, respectively. Similar trends in pharmacodynamic effects were also observed.
Description
Rivaroxaban, a factor Xa inhibitor, is the active ingredient in RIVAZEST Tablets. Each RIVAZEST tablet contains 2.5 mg, 10 mg, 15 mg, or 20 mg of rivaroxaban. The inactive ingredients of RIVAZEST are: microcrystalline cellulose, lactose monohydrate, hypromellose, croscarmellose sodium, sodium lauryl sulfate, and magnesium stearate. Additionally, the proprietary film coating mixture used is macrogol, hypromellose titanium dioxide and red iron oxide.
Packaging Information
RIVAZEST 2.5……. Blister pack of 14 Tablets
RIVAZEST 10……. Blister pack of 14 Tablets
RIVAZEST 15……. Blister pack of 14 Tablets
RIVAZEST 20……. Blister pack of 14 Tablets
Storage and Handling Instructions
Store at 25°C (77°F) or room temperature; excursions permitted to 15°-30°C (59°-86°F). Keep out of the reach of children.
Patient Counselling Information
Instructions for Patient use
- Advise patients to take RIVAZEST only as directed.
- Remind patients to not discontinue RIVAZEST without first talking to their healthcare professional.
- Advise patients with atrial fibrillation to take RIVAZEST once daily with the evening meal.
- Advise patients for initial treatment of DVT and/or PE to take RIVAZEST 15 mg or 20 mg tablets with food at approximately the same time every day.
- Advise patients who are at a continued risk of recurrent DVT and/or PE after at least 6 months of initial treatment, to take RIVAZEST 10 mg once daily with or without food.
- Advise patients who cannot swallow the tablet whole to crush rivaroxaban tablets and combine with a small amount of applesauce followed by food.
- For patients requiring an NG tube or gastric feeding tube, instruct the patient or caregiver to crush the rivaroxaban tablet and mix it with a small amount of water before administering via the tube.
- If a dose is missed, advise the patient to take rivaroxaban as soon as possible on the same day and continue the following day with their recommended daily dose regimen.
Bleeding Risks
- It is necessary to report any unusual bleeding or bruising to the physician. It might take them longer than usual to stop bleeding, and that they may bruise and/or bleed more easily when they are treated with rivaroxaban.
- If patients have had neuraxial anesthesia or spinal puncture, and particularly, if they are taking concomitant NSAIDs or platelet inhibitors, advise patients to watch for signs and symptoms of spinal or epidural hematoma, such as back pain, tingling, numbness (especially in the lower limbs), muscle weakness, and stool or urine incontinence. If any of these symptoms occur, advise the patient to contact the physician immediately.
Like all medicines, rivaroxaban can cause side effects, although not everybody gets them.
Like other similar medicines (antithrombotic agents), rivaroxaban may cause bleeding which may potentially be life threatening. Excessive bleeding may lead to a sudden drop in blood pressure (shock). In some cases the bleeding may not be obvious.
Possible Side Effects which may be a Sign of Bleeding
Tell your doctor immediately if you experience any of the following side effects:
- long or excessive bleeding
- exceptional weakness, tiredness, paleness, dizziness, headache, unexplained swelling, breathlessness, chest pain or angina pectoris, which may be signs of bleeding.
Your doctor may decide to keep you under closer observation or change how you should be treated.
Possible Side Effects which may be a Sign of Severe Skin Reaction
Tell your doctor immediately if you experience skin reactions such as:
- spreading intense skin rash, blisters or mucosal lesions, e.g. in the mouth or eyes (Stevens Johnson syndrome/toxic epidermal necrolysis). The frequency of this side effect is very rare (up to 1 in 10,000).
- a drug reaction that causes rash, fever, inflammation of internal organs, hematologic abnormalities and systemic illness (DRESS syndrome). The frequency of this side effect is very rare (up to 1 in 10,000).
Possible Side Effects which may be a Sign of Severe Allergic Reactions
Tell your doctor immediately if you experience any of the following side effects related to bleeding.
Invasive or Surgical Procedures: Instruct patients to inform their healthcare professional that they are taking rivaroxaban before any invasive procedure (including dental procedures) is scheduled.
Concomitant Medication and Herbals: Advise patients to inform their physicians and dentists if they are taking, or plan to take, any prescription or over-the-counter drugs or herbals, so their healthcare professionals can evaluate potential interactions.
Pregnancy and Pregnancy-related Hemorrhage
- Advise patients to inform their physician immediately if they become pregnant or intend to become pregnant during treatment with rivaroxaban
- Advise pregnant women receiving rivaroxaban to immediately report to their physician any bleeding or symptoms of blood loss.
Lactation: Advise patients to discuss with their physician the benefits and risks of rivaroxaban for the mother and for the child if they are nursing or intend to nurse during anticoagulant treatment
Females and Males of Reproductive Potential: Advise patients who can become pregnant to discuss pregnancy planning with their physician.
References
1. Mekaj YH, Mekaj AY, Duci SB, et al. New oral anticoagulants: their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther Clin Risk Manag. 2015; 11: 967–977.
2. Weitz JI, Lensing AWA, Prins MH, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376:1211-22.
3. Haas S, Bode C, Norrving B, et al. Practical guidance for using rivaroxaban in patients with atrial fibrillation: Balancing benefit and risk. Vascular Health and Risk Management. 2014:10 101–114.
4. Perzborn E, Roehrig S, Straub A, et al. Rivaroxaban: A new oral Factor Xa inhibitor. Arterioscler Thromb Vasc Biol. 2010;30:376-381.
5. Iram F, Iqbal A and Husain A. A review on rivaroxaban: A prominent oral anti-coagulant agent. Int J Pharma Chemical Res. 2015;1(3):140-148.
6. Samama MM. The mechanism of action of rivaroxaban--an oral, direct Factor Xa inhibitor--compared with other anticoagulants. Thromb Res. 2011;127(6):497-504.
7. Mueck W, Schwers S and Stampfuss J. Rivaroxaban and other novel oral anticoagulants: Pharmacokinetics in healthy subjects, specific patient populations and relevance of coagulation monitoring. Thrombosis Journal. 2013;11:10.
8. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
9. The Executive Steering Committee. Rivaroxaban—Once daily, oral, direct factor xa inhibition compared with vitamin k antagonism for prevention of stroke and embolism trial in atrial fibrillation: rationale and design of the ROCKET AF study. Am Heart J. 2010;159:340-347.e1.
10. Lee H, Chan Y, Tu H, et al. The effectiveness and safety of low-dose rivaroxaban in Asians with non-valvular atrial fibrillation. Int J Cardiol. 2018;261:78–83.
11. Schiavoni M, Margaglione M and Coluccia A. Use of dabigatran and rivaroxaban in non-valvular atrial fibrillation: One-year follow-up experience in an Italian centre. Blood Transfus. 2018;16:209-214.
12. The EINSTEIN investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499-2510.
13. The EINSTEIN–PE Investigators*. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287-1297.
14. Weitz JI, Lensing AWA, Prins MH, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376:1211-22.
15. Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765-2775.
16. Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. The Lancet. 2008;372(9632):31–39.
17. Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. The New England Journal of Medicine. 2008;358(26):2776–2786.
18. Spyropoulos AC, Ageno W, Albers GW, et al. Post-discharge prophylaxis with rivaroxaban reduces fatal and major thromboembolic events in medically ill patients. J Am Coll Cardiol. 2020;75(25):3140–3147.
19. Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319-30.
20. Branch KR, Probstfield JL, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with heart failure and chronic coronary or peripheral artery disease: The COMPASS Trial. Circulation. 2019;140(7):529-537.
21. Cardiovascular outcomes for people using anticoagulation strategies – COMPASS. Available at: https://www.acc.org/latest-in-cardiology/clinical-trials/2017/08/26/02/19/compass 2020 July 10.
22. Prescribing information of RIVAZEST as accessed on 31st Oct 2020.