SULBACIP
Sulbactam Sodium I.V. 1 gm & 2gm
Make Your Own Power
Product Monograph
Accumulating resistance among Gram-negative pathogens, especially Pseudomonas and
Acinetobacter, has spurred the search for novel agents, but the results are disappointing to date, with
no anti-Gram-negative drug possessing a truly novel mode of action submitted for registration in more than 30
years. 1
A renewed interest in sulbactram as a true
beta-lactam antibiotic 3 has been noted recently, owing to its intrinsic activity against the
Acinetobacter spp., including carbapenem-resistant isolates.
To meet the present challenges, it is, therefore, re-examine important older to ムforgotten' compounds that
may prove useful. Reviving polymyxins is one example of this approach; others include a renewed interest in
intravenous (I.V.) fosfomycin and, recently, I.V. sulbactam alone.
Developed and first marketed in the 1980s, sulbactam was traditionally known as a beta-lactam sulphone that
exhibits a synergistic effect in combination with a large number of beta-lactam antibiotics. This synergy is
related mainly to the beta-lactamase inhibitory properties of sulbactam. 2
Over the past decade, the proportion of hospital-acquired infections caused by Acinetobacter spp. has
increased worldwide. In the National Nosocomial Infections Surveillance System (NNISS) among Gram-negative
bacilli isolated in intensive care unit (ICU) patients with pneumonia, Acinetobacter 3 spp.
ranked fourth (6.9%) during the study period, and it was the only Gram-negative organism with a
significant increase in prevalence in the same
years.
The first report of Acinetobacter from India dates back to 1963. In a recent article, 4
published in J Antimicrobial Chemotherapy 2011, a 10-year retrospective analysis of blood cultures conducted by
Neeraj Goel et al. in a hospital reported an increasing trend in the incidence of multidrug-resistant (MDR)
Acinetobacter spp., including carbapenem-resistant isolates. A similar report on the high prevalence of
MDR Acinetobacter baumannii (A.baumannii), has been reported from various hospitals across India
5,6,7,8 nevertheless with a difference in the susceptibility pattern.
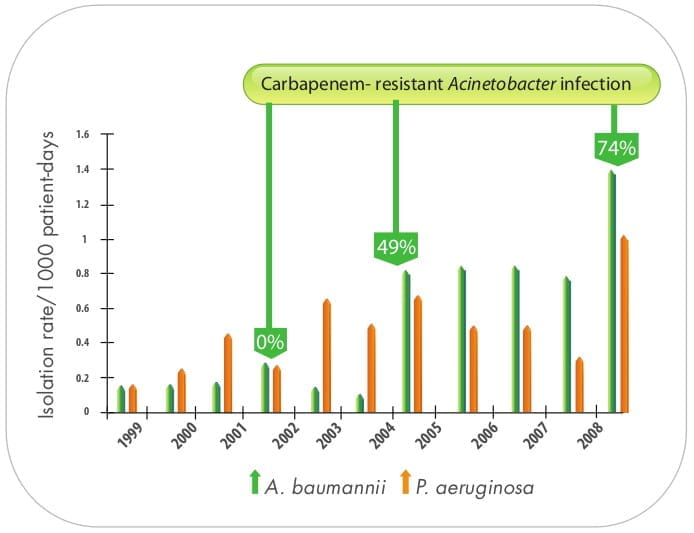
Though the effectiveness of carbapenems, which used to be the antimicrobials of choice for A.baumannii,
have been increasingly compromised and no longer constitute salvage therapy in many parts of world, including
India, it is anticipated that the addition of other active antimicrobials to carbapenem 9 may salvage
its role as a core antimicrobial to fight against MDR Acinetobacter. In this regard, polymyxins have
played a pivotal role against Acinetobacter infections.
Sulbactam seems to be another interesting agent. A series of in vitro 9,10 and in vivo
11,12 animal studies have reported encouraging results proving the intrinsic activity of
sulbactam against Acinetobacter. Moreover, it has been proposed that an optimal dose of at least
>6-8 gm 13 of the sulbactam compound in divided doses, assuming normal renal function, should be
administered per day.
A disadvantage is that, in a number of countries, sulbactam is commercially available only in combination with
ampicillin or cefoperazone at a fixed 2:1 ratio.
SULBACIP I.V. 1 gm
Each vial contains
Sulbactam Sodium USP equivalent to Sulbactam .... 1 gm
SULBACIP I.V. 2 gm
Each vial contains
Sulbactam Sodium USP equivalent to Sulbactam .... 2 gm
Powder for reconstitution (I.V)
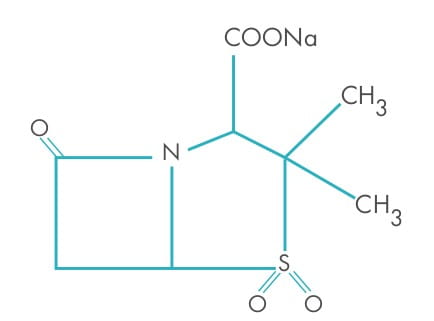
Sulbactam is a derivative of the basic penicillin nucleus. Chemically, sulbactam sodium is sodium penicillinate
3-dimethyl-7-oxo-4-thia-1-azabicyclo sulphone; [3.2.0] sodium (2S, 5R)-3, heptanes-2-carbaoxylate 4, 4-dioxide. Its
chemical formula is C
8H
10NNaO
5S, with a molecular weight of 255.22. It contains
92 mg sodium (4 mEq) per gram.
14
Sulbactam has been traditionally known as a synthetic beta-lactamase inhibitor. However, a feature that
distinguishes sulbactam from other available beta-lactamase inhibitors is its direct antimicrobial activity
against the Acinetobacter spp. and Bacteroides fragilis, against which most cephalosporins
display little or no activity. 10
This activity is mediated by its interaction with penicillin-binding proteins 2 (PBPs), rather than an
inhibitory effect on beta-lactamases. An early report by Urban et al. illustrated the clinical utility of
sulbactam during an outbreak of Acinetobacter spp. resistant to carbapenems, aminoglycosides and other
beta-lactams. 15
Rondriguez-Hernandez et al., in an experimental pneumonia model in mice using susceptible A. baumannii
strains, demonstrated that sulbactam was as efficacious as imipenem in terms of sterility of the lungs and in
the bacterial clearance from the lungs and blood, provided that the time above the minimum inhibitory
concentration (MIC) for sulbactam (1.84 hours) was similar to that for imipenem (2.01 hours). 16
Several in vitro and in vivo animal studies have provided evidence of intrinscic activity 12,15,16
against Acinetobacter isolates and improved survival with sulbactam. 11
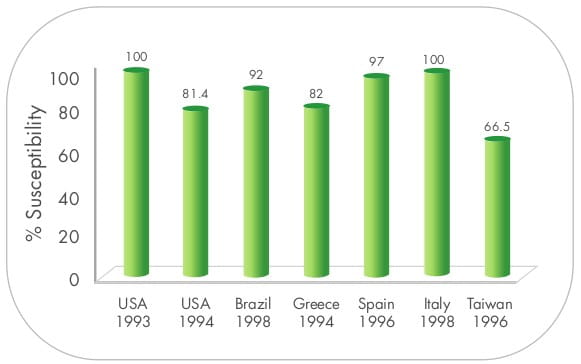
Additionally, Yoshiki Obana and Takeshi Nishino have demonstrated that sulbactam alone was very active against 40
clinical isolates of A.calcoaceticus and inhibited the growth of all the strains at concentrations
<3.13 mg/L. 12
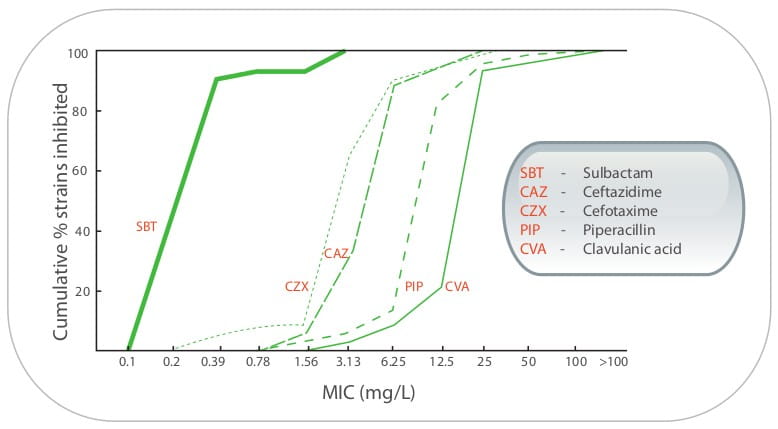
Although sulbactam seems to be promising, based on in vitro susceptibility and in vivo animal
models, several authors discourage the use of sulbactam as a monotherapy. A series of data that comes from
in vitro and animal models have shown enhanced activity when sulbactam is combined with cefepime,
17,18 imipenem, 19 meropenem, 9 colistin, 20 amikacin, 21
rifampicin 22 and ticarcillin-clavulanate 11 Such enhanced activity may also be retained
for infections with sulbactam and carbapenem-non-susceptible Acinetobacter isolates.
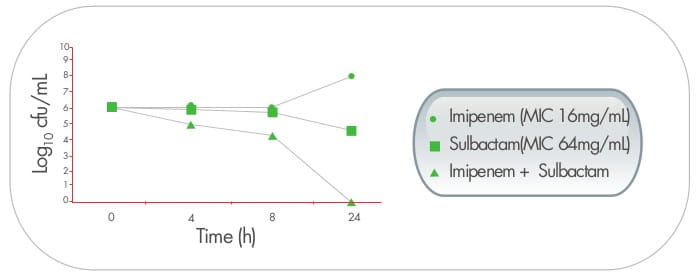
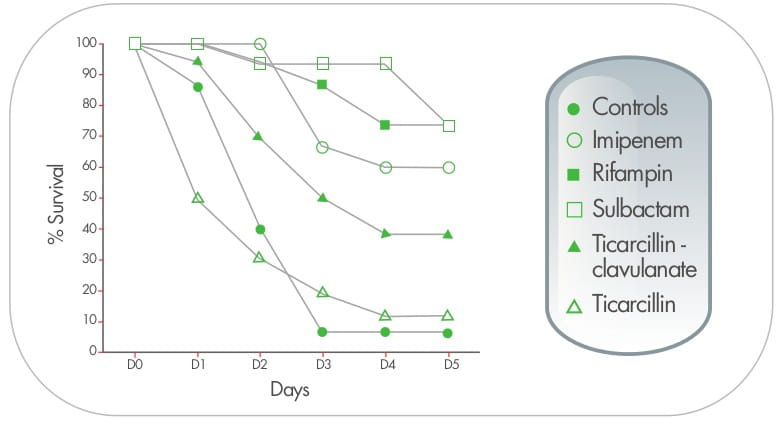
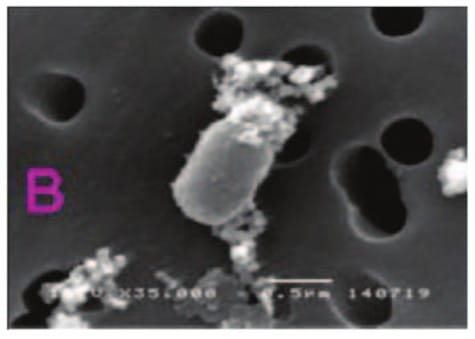
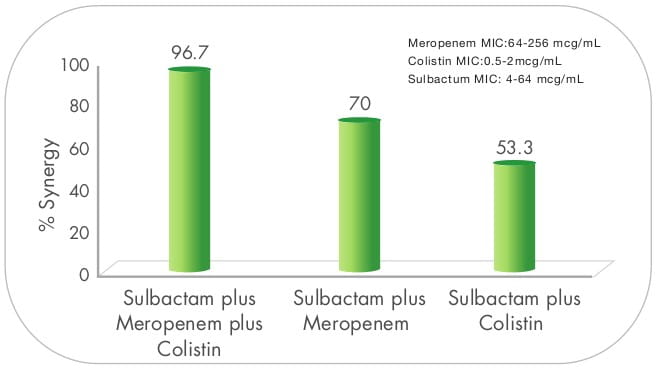
In a study from Thailand, triple dosing combination of meropenen plus sulbactam plus colistin proved to be highly
synergistic against MDR A. baumannii including, imipenem and meropenem - resistant strains.The effect
was also observed in scanning electron micrographs, as demonstrated by cellular disruption and release of intra
cellular material. 9

It is anticipated that sulbactam alters the permeability of the outer membrane of Gram-negative bacilli,
resulting in the leakage of beta-lactamases and, thus, better penetration by other antibacterial agents.
23
Although there is limited data available on the optimal dosing of sulbactam, recent literature recommends the use
of high-dose sulbactam (minimum of 6 gm/day up to 12 gm/day, 15 assuming normal renal function) in
combination with other antibiotics for severe infections or those involving carbapenem-resistant
Acinetobacter strains. 24 The high-dosing regimen selection was based upon experimental
studies and the knowledge that doses of sulbactam >200 mg/kg/day were found to be toxic in animals.
25
Lister et al., comparing two-dose (40 mg/kg and 80 mg/kg) regimens, 23 showed a dose- dependent
reduction of bacterial count with the highly resistant strain (MIC >128/64 mcg/mL) in the mouse model of
bacteraemia.
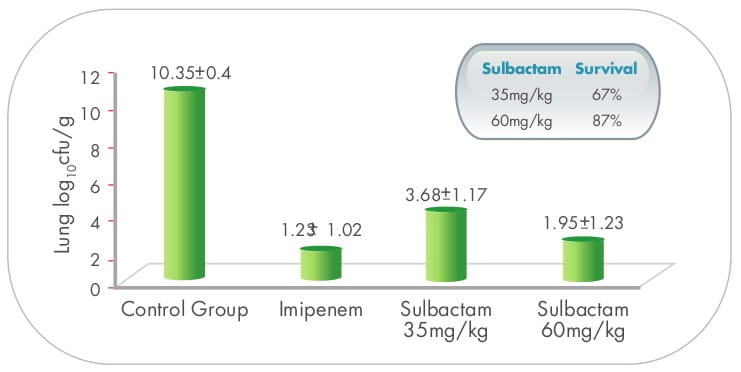
In the pneumonia model, sulbactam (35 mg/kg) improved the survival and the sterilization of the lungs and blood,
compared to the controls. However, the results were significantly worse than those obtained with imipenem using
a similar dose. When the dose of sulbactam was increased (60 mg/kg), reaching a t>MIC similar to that of
imipenem (1.84 versus 2.01 hours), sulbactam was as efficacious as imipenem, 16,11 suggesting
time-dependent activity.
Recent literature suggests use of atleast 6 gms/day in 3 equal divided doses of sulbactam against MDR
Acinetobacter.
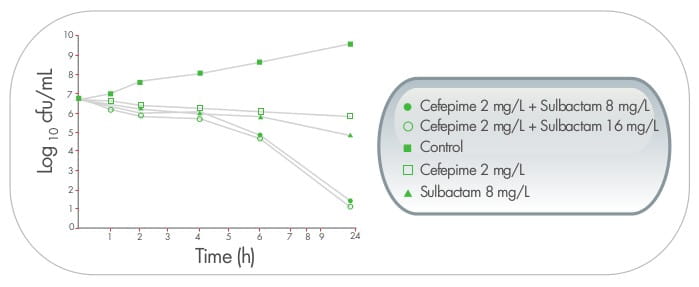
In another time-kill study, 26 sulbactam at a concentration of 1x MIC (8 mg/mL), when used in
combination with meropenem, resulted in a sustained synergistic bactericidal effect lasting for at least 48
hours.
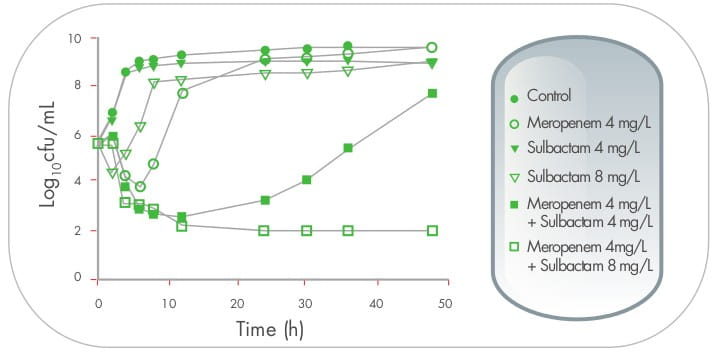
In the same study, when the combination of sulbactam and meropenem was administered to the BALB/c mice infected
intraperitoneally with Acinetobacter spp., survival rates were significantly higher than those treated
with meropenem alone. 26
Addition of high-dose sulbactam results in 16-fold
reductions in imipenem or meropenem MIC vaues against Acinetobacter isolates, and improves survival.
16
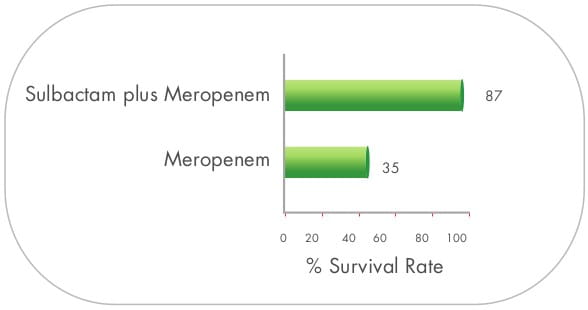
High dose MDR A.baumannii ventilator-associated pneumonia (VAP)
In a prospective, randomized trial conducted by a group of investigators from Greece, a high- dose regimen of
sulbactam was assessed for MDR Acinetobacter. A total of 27 patients were enrolled in the study.
Patients were randomly assigned to one of two treatment regimens, i.e., 9 gm daily doses (group A) and 12 gm
daily doses (group B) of sulbactum. The duration of therapy was 8 ± 2 days for both groups.
Clinical improvement was seen in 66.7% of the study population (64.3% of group A patients and 69.2% of group B
patients, respectively). Bacteriological success was achieved in 77.8% of the study population. The mortality
rates did not differ significantly between the two groups. No major adverse reactions were recorded with the use
of a high-dose regimen of sulbactam. 25
Sulbactam plus carbapenem therapy for carbapenem-resistant A.baumannii bacteraemia
In 4 patients infected with A.baumannii resistant to commonly available antibiotics, including
carbapenem and sulbactam, a combination of carbapenem and sulbactam (1 gm/6h) led to favorable clinical outcomes
in all the patients. Moreover, 16-fold reductions in imipenem or meropenem MIC values were observed when either
of these agents was combined with sulbactam at a fixed concentration of 8 mcg/mL. This sulbactam concentration
was arbitrarily chosen for the in vitro study because of pharmacokinetic data showing that, after I.V.
administration of sulbactam 1 gm, serum sulbactam levels remain above 8 mcg/mL for 2-5 hours in healthy adults.
As both antimicrobials are time-dependent, the therapeutic benefit of combination therapy is probably related to
the amount of time in which serum carbapenem concentrations exceed the MIC. This MIC is expected to decline in
the presence of an easily achievable serum level of sulbactam. 27
A case report 28 of intractable severe bacteraemia due to carbapenem-resistant A.baumannii
also supported the use of high-dose sulbactam.
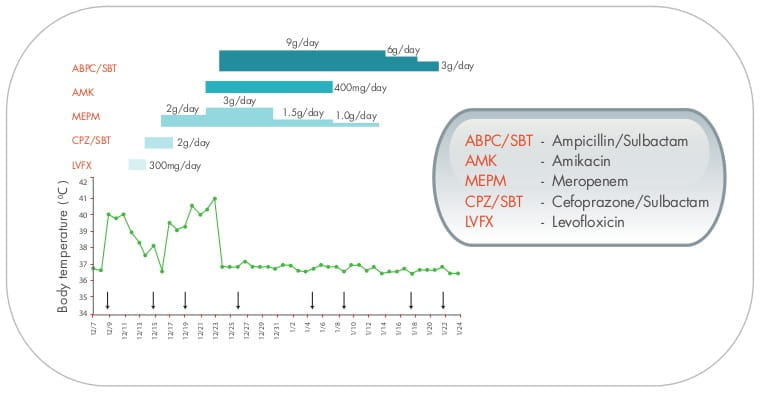
The study investigator concluded that the patientメs improvement may have been related to a synergistic effect of
sulbactam with other antibiotics and not due to sulbactam alone.
Sulbactam - Reasonable empirical therapy in institutions with highly endemic MDR A.baumannii.
In this retrospective study of 93 patients, ampicillin/sulbactam was administered as empirical therapy in 65% of
patients with MDR A.baumannii, while in non-MDR cases, it was prescribed according to the
susceptibility report. Among severely ill patients, ampicillin-sulbactam therapy significantly decreased the
risk of death (P=0.02, odds ratio =7.64). This data, although not in a case control design, suggest that
ampicillin/sulbactam treatment, in combination with another antibiotic for MDR A. baumannii
bacteraemia, is as effective as appropriate conventional mono- or combination-therapy for A. baumannii
bacteraemia involving susceptible strains. 29
Sulbactam/ampicillin as effective as carbapenem against A.baumannii.
In two different studies, i.e., bacteraemia 30 (79 patients) and VAP 31 (75 patients)
caused due to A.baumannii, sulbactam/ampicillin proved to be as effective as imipenem/cilastatin.
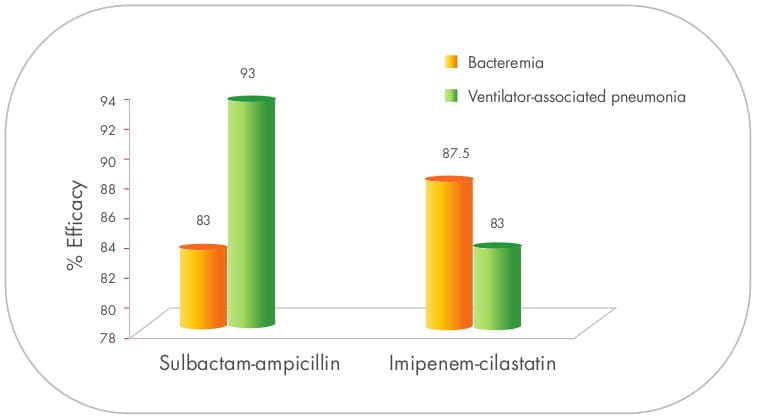
No statistically significant differences between the groups were noted with regard to associated mortality,
duration of mechanical ventilation or length of stay in the ICU or hospital. However, adjunctive aminoglycoside
therapy was used more often in the sulbactam/ampicillin group.
MDR A.baumannii meningitis
In eight cases of nosocomial A.baumannii meningitis, sulbactam at a dose of 1 gm every 6 hours cured 6
patients while 2 patients died of meningitis. There were no side effects with the ampicillin/sulbactam
treatment. All the patients had fever, neck stiffness or meningeal signs and a low consciousness level; also, in
their cerebrospinal fluid (CSF), pleocytosis, a low glucose level and an elevated protein level were noted.
In conclusion, sulbactam proved to be effective therapy for meningitis caused by A. baumannii resistant
to imipenem and other beta-Iactam drugs. 32
In a single case report 33 of a patient infected with MDR A.baumannii , 4-fold to 32-fold
increases in peak or trough CSF bactericidal titre and serum bactericidal titre were observed in three-drug
therapy (meropenem [2 gm/8 hours], MIC = 256 mg/L plus sulbactam [1 gm/8 hours], MIC=128 mg/L plus I.V. colistin
[2.5 mg/kg 12 hours] and intraventricular [5 mg/day], MIC= 1 mg/L)) when comparing to those in a two-drug
therapy period.
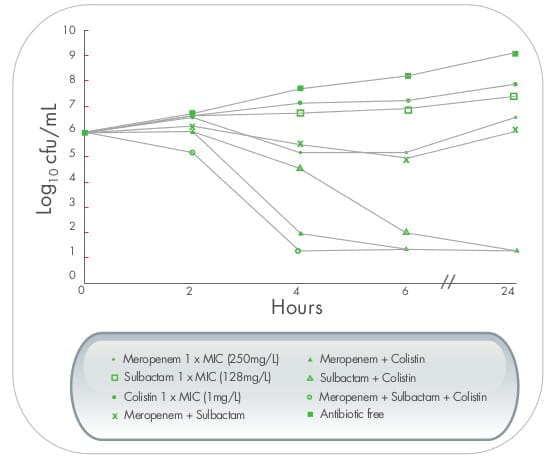
Thus, the combined use of colistin with meropenem and/sulbactam may provide good therapeutic options, even though
meropenem and sulbactam show in vitro resistance to MDR A.baumannii.
Despite the lack of well controlled clinical studies, sulbacam in combination with other antibiotics
seems to be a reliable option for the treatment of MDR A .baumannii.
There is limited data on the undesirable effects with sulbactam alone. However, sulbactam in combination with
ampicillin and cefoperazone has been generally well tolerated. As with other antibiotics, the most frequent side
effects observed with sulbactam when used in combination have been gastrointestinal, such as diarrhoea/loose
stools (3.09%), and nausea and vomiting (0.6%). Additional systemic reactions reported in less than 1% of the
patients were itching, candidiasis, fatigue, malaise, headache, chest pain, flatulence, abdominal distension,
glossitis, urine retention, dysuria, oedema, facial swelling, erythema, chills, tightness in the throat,
substernal pain, epistaxis, and mucosal bleeding.
Laboratory changes most commonly reported involved elevated hepatic enzymes (AST, ALT, alkaline phosphatase,
lactate dehydrogenase). Haematological abnormalities (decreased haematocrit/haemoglobin, leucopenia,
lymphopenia, thrombocytopenia or increased lymphocytes, monocytes, basophils, eosinophils and platelets),
decreased albumin and total proteins, increased creatinine, and the presence of red blood cells and hyaline
casts in the urine are less frequent.
However, in a small group of studies and/or case reports published till date using 10-12 gm/day 34,35
of sulbactam, no major adverse effects were observed. In one study, patients were randomly assigned to receive 9
gm/day sulbactam or colistin 9 million international units (MIU)/day in three equal divided doses. A total of 28
patients were enrolled. Resolution of symptoms and signs occurred in 60% of the colistin group and 61.5% of the
sulbactam group. Mortality rates (14 days and 28days) were 15.3% and 30% for the sulbactam group and 20% and 33%
for the colistin group, respectively. Adverse events were 39.6% (including 33% nephrotoxicity) for the colistin
group and 30.7% (15.3% nephrotoxicity) for the sulbactam group (P=NS).
Another small group of 40 consecutive patients with nosocomial infections caused by MDR A. baumannii
were treated with I.V. sulbactam. The median daily dose of sulbactam was 3 gm and 6 patients received 6 gm.
The infections were primary bloodstream (32.5%), pneumonia (30%), urinary tract (15%), peritonitis (7.5%),
surgical site (7.5%) and meningitis (5%). Most were severe infections with underlying conditions (median APACHE
II score: 14.5) and 72.5% occurred in the ICU.
While 27 patients (67.5%) were improved/cured, 7 (17.5%) were failures and 6 (15%) were considered to have an
indeterminate outcome because patients died within the first 48 hours of treatment.
No adverse effects were observed. This study indicates that sulbactam at a dose of 6 gm/day is a safe 36
therapeutic option to treat severe nosocomial infections caused by MDR A. baumannii.
SULBACIP I.V. is indicated for the treatment of some serious infections caused by the following:
- MDR /Carbapenem resistant Acinetobacter
- Extended Spectrum Beta-Lactamases infections (ESBLs)
Where sensitivity testing suggests that they are caused by susceptible bacteria, including those of the lower
respiratory tract, bacteraemia/sepsis, meningitis, surgical wounds and the urinary tract, when more commonly
used systemic antibacterial agents may be contraindicated or may be ineffective because of bacterial resistance.
Sulbacip I.V should always be used in combination with other antibiotics.
The pharmacokinetic profile of sulbactam is significantly altered by haemodialysis. The dosing should be
scheduled to follow a dialysis period.
Hepatic Impairment
No data is available on sulbactam alone; however, when used with ampicillin at I.V. doses of 3-9 gm/day in
patients with chronic liver disease, the adverse effects observed were minor (oral candidiasis and local
injection site pain) and infrequent; blood chemistry tests were unchanged following therapy.
Pregnancy
Reproduction studies of sulbactam in combination with ampicillin and cefoperazone have been performed in rats at
doses up to 10 times the human dose and have revealed no evidence of impaired fertility and no teratogenic
findings. Sulbactam is known to cross the placental barrier. So, it should be used during pregnancy only if
clearly needed.
Lactation
Although only small quantities of sulbactam are excreted in human milk, caution should be exercised when
sulbactam is administered to a lactating nursing mother.
Method of I.V. Preparation & Administration
Reconstitution:
SULBACIP I.V. 1 gm
Reconstitute with 6.2 mL of 5% Dextrose, 0.9% Sodium Chloride Injection or Sterile Water for Injection, then
further dilute to 20 mL of the same solution.
SULBACIP I.V. 2 gm
Reconstitute with 12.4 mL of 5% Dextrose, 0.9% Sodium Chloride Injection or Sterile Water for Injection, then
further dilute to 40 mL of the same solution.
SULBACIP I.V. should be administered over a period of 15-60 minutes.When concomitant therapy with other
antibacterials is indicated, SULBACIP I.V. should be reconstituted and administered separately.
For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only OR for Specialist Use
only.
Sulbactam Sodium for Intravenous (I.V) Use
Sulbacip I.V. 1 gm
Each vial contains:
Sulbactam Sodium USP equivalent to Sulbactam .... 1 gm
Sulbacip I.V. 2 gm
Each vial contains:
Sulbactam Sodium USP equivalent to Sulbactam .... 2 gm
Powder for reconstitution (I.V.)
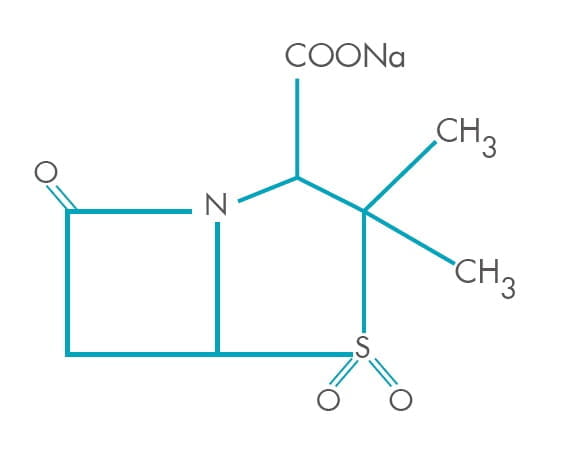
Sulbactam is a derivative of the basic penicillin nucleus. Chemically, sulbactam sodium is sodium penicillinate
3-dimethyl-7-oxo-4-thia-1-azabicyclo sulphone; [3.2.0] sodium (2S, 5R)-3, heptanes-2-carbaoxylate 4, 4-dioxide.
Its chemical formula is C 8H 10NNaO 5S, with a molecular weight of 255.22. It
contains 92 mg sodium (4 mEq) per gram. Its structural formula is as below:
Sulbactam is a synthetic beta-lactamase inhibitor. A feature that distinguishes sulbactam from other available
beta-lactamase inhibitors is its direct antimicrobial activity against the Acinetobacter spp. and
Bacteroides fragilis.
Sulbactam is a synthetic beta-lactamase inhibitor. A feature that distinguishes sulbactam from other available
beta-lactamase inhibitors is its direct antimicrobial activity against the Acinetobacter spp. and
Bacteroides fragilis.
Its mechanism of antimicrobial activity against Acinetobacter baumannii (A.baumannii) strains is related
to its intrinsic affinity for the essential penicillin-binding proteins 2 (PBPs) of these organisms, and for
altering the permeability of the outer membrane of Gram-negative bacilli, resulting in the leakage of
beta-lactamases and, thus, better penetration by other antibacterial agents.
Many data that comes from in vitro and animal models, which show enhanced activity when sulbactam is
combined with cefepime, imipenem, meropenem, colistin, amikacin, rifampicin and ticarcillin-clavulanate. Such
enhanced activity may also be retained for infections with sulbactam & carbapenen non-susceptible
Acinetobacter.
Absorption
After a 30-minute infusion of 1 gm of sulbactam, a peak concentration of approximately 43 mcg/mL is obtained.
Multiple dosing at rates as high as 500 mg every 6 hours (intramuscular [I.M.]) or 1gm twice a day (1-hour
infusions or I.V. bolus) does not appear to lead to accumulation of the drug as indicated by an increase in the
peak serum concentrations or the area under the curves (AUCs).
Distribution
The binding of sulbactam to human serum proteins is approximately 38%. In a two-compartment pharmacokinetic
model, the apparent volume of distribution of the central compartment of 9 to 16 litres is in the range of total
extracellular fluid in humans (approximately 15 litres) and suggests that sulbactam is widely distributed in the
extracellular fluid.
The total apparent volume of distribution of between 19 and 28 litres is over half that of the total body fluid,
approximately 40 litres in a 70 kg human, suggesting that sulbactam may be widely distributed into the tissues.
An hour after I.V. administration of 1 gm sulbactam (at its peak concentration in serum) in 8 patients with
bacterial respiratory infections, a penetration ratio (alveolar lavage fluid versus serum) of 61% has been
reported. In patients with inflamed meninges, the cerebrospinal fluid (CSF) penetration of sulbactam is reported
to be 2-32% of serum levels, which are approximately 42-60 mg/L after a 1 gm I.V. dose. After 500 mg of a
parenteral dose, appreciable concentrations were observed in the peritoneal fluid (14 mg/L), intestinal mucosa
(0-28 mg/gm), prostate (7 mg/gm) and pus (12.7 mg/L).
Metabolism and Excretion
The mean serum half-life is approximately 1 hour in healthy volunteers. Approximately 70% of a parenteral dose of
sulbactam was excreted in the urine between 0 and 6 hours, with an additional recovery of 5% between 6 and 12
hours post-dose. The renal clearance is approximately 204 mL/min and was not dose-dependent. The total clearance
of drug from the serum was 266 mL/min. The non-renal clearance was 65 mL/min.
An additional similarity to the beta-lactam antibiotics is the increase of approximately 40% in the half-life of
sulbactam in humans due to a prior dose of probenecid.
Use in Renal Impairment
In patients with different degrees of renal function who were administered sulbactam/cefoperazone, the total body
clearance of sulbactam was highly correlated with the estimated creatinine clearance. Patients who were
functionally anephric showed a significantly longer half-life of sulbactam (mean 6.9 and 9.7 hours in separate
studies). Haemodialysis removes 30% of the given doses of sulbactam and, hence, supplemental doses are
recommended after dialysis.
Use in Hepatic Impairment
Sulbactam in combination with ampicillin has been administered safely in I.V. doses of 3-9 gm/day in patients
with chronic liver disease. Adverse effects observed were minor (oral candidiasis and local injection site pain)
and infrequent; blood chemistry tests were unchanged following therapy.
Use in Paediatric Patients (2 to 14 years of age)
Single doses of 12.5 or 25 mg/kg infused over 3 minutes results in mean peak plasma concentrations of 71 or 163
mcg/mL after 5 minutes of dosing. The mean terminal-phase half-life was 1.75 hours, the mean total plasma
clearance was 180 mL/min/1.73 m2, and the mean apparent volume of distribution was 340 mL/kg. Approximately
70-80% of an I.V. dose was excreted unchanged in the urine. In children with cystic fibrosis, both the total
plasma clearance and the apparent volume of distribution were significantly increased.
Use in Geriatric Patients
Comparative pharmacokinetic data for sulbactam in young and elderly individuals suggest that there is a
prolongation of antimicrobial activity as age increases, which is due to the area under the serum
concentration-time curve, half-life, serum maximum concentration and decreased total clearance in the older age
group.
SULBACIP I.V. is indicated in the treatment of the following infections, where sensitivity testing
suggests that they are caused by susceptible bacteria:
Treatment of some serious infections caused by multidrug-resistant (MDR) Acinetobacter and ESBLs infections,
including those of the lower respiratory tract, bacteraemia/sepsis, meningitis, surgical wounds and the urinary
tract, when more commonly used systemic antibacterial agents may be contraindicated or may be ineffective
because of bacterial resistance.
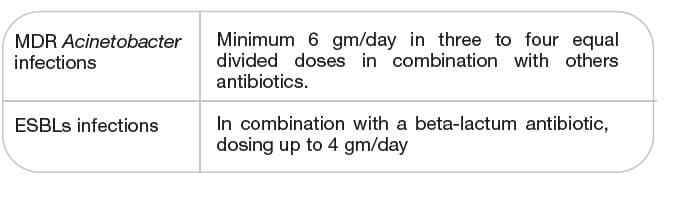
Adults with normal renal function:
- For serious Acinetobacter infections, minimum of 6 gm/day of SULBACIP I.V. in three to four equal
divided doses in combination with other antibiotics, is recommended.
- For ESBLs infections when used in combination with a beta-lactam antibiotic, dosing up to 4 gm/day of
SULBACIP I.V., is recommended.
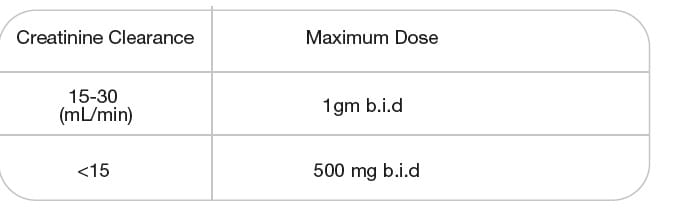
Renal Impairment
Dosage regimens of sulbactam should be adjusted in patients with marked decrease in renal function (creatinine
clearance of less than 30 mL/min) to compensate for the reduced renal clearance of sulbactam.
The pharmacokinetic profile of sulbactam is significantly altered by haemodialysis. The dosing should be
scheduled to follow a dialysis period.
Paediatric Use
The recommended dose of sulbactam when administered along with cefoperazone and ampicillin is 80-100 mg/kg/day in
two to four equally divided doses.
I.V. Administration Reconstitution
SULBACIP I.V. 1 gm
Reconstitute with 6.2 mL of 5% Dextrose, 0.9% Sodium Chloride Injection or Sterile Water for Injection, then
further dilute to 20 mL of the same solution.
SULBACIP I.V. 2 gm
Reconstitute with 12.4 mL of 5% Dextrose, 0.9% Sodium Chloride Injection or Sterile Water for Injection, then
further dilute to 40 mL of the same solution.
SULBACIP I.V. should be administered over a period of 15-60 minutes.
Sulbactam is contraindicated in patients with a known allergy/hypersensitivity to beta-lactam antibiotics.
Before therapy with SULBACIP I.V. is initiated, careful inquiry should be made concerning
previous hypersensitivity reactions and other allergens. Hypersensitivity reactions, including skin rash,
urticaria, pruritus, fever and anaphylaxis, have been observed. If allergic reactions occur, SULBACIP
I.V. should be discontinued and the appropriate therapy should be instituted. In case of severe
anaphylactic reactions occur with Sulbacip I.V. use, it requires immediate emergency treatment
with epinephrine. Oxygen, I.V. steroids and airway management, including intubation, should be administered as
indicated.
Treatment with antibacterial agents alters the normal flora of the colon, leading to the overgrowth of
Clostridium difficile ( C.difficile). If C.difficile-associated diarrhoea (CDAD) is suspected
or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued.
Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of
C.difficile, and surgical evaluation should be instituted as clinically indicated.
As with any potent systemic agent, it is advisable to check periodically for organ system dysfunction during
extended therapy; this includes the renal, hepatic and haematopoietic systems. This is particularly important in
neonates, especially when premature, and other infants.
Drug Interactions
Probenecid decreases the renal tubular secretion of sulbactam. Concurrent use of probenecid may result in
increased and prolonged blood levels of sulbactam.
Renal Impairment
Please refer under Pharmacology and Dosage and Administration.
Hepatic Impairment
No data is available on sulbactam alone; however, when used with ampicillin at I.V. doses of 3-9 gm/day in
patients with chronic liver disease, adverse effects observed were minor (oral candidiasis and local injection
site pain) and infrequent; blood chemistry tests were unchanged following therapy.
Pregnancy
Reproduction studies of sulbactam in combination with ampicillin and cefoperazone have been performed in rats at
doses up to 10 times the human dose and have revealed no evidence of impaired fertility and no teratogenic
findings. Sulbactam is known to cross the placental barrier. There are, however, no adequate and well-controlled
studies in pregnant women. Because animal reproduction studies are not always predictive of human response,
sulbactam should be used during pregnancy only if clearly needed.
Lactation
Although only small quantities of sulbactam are excreted in human milk, caution should be exercised when
sulbactam is administered to a lactating mother.
Paediatric Use
Please refer under Pharmacology and Dosage and Administration.
Geriatric Use
Please refer under Pharmacology and Dosage and Administration.
There is limited data on undesirable effects with sulbactam alone. However, sulbactam in combination with
ampicillin and cefoperazone has been generally well tolerated. As with other antibiotics, the most frequent side
effects observed with sulbactam when used in combination have been gastrointestinal, such as diarrhoea/loose
stools (3.09%), and nausea and vomiting (0.6%). Additional systemic reactions reported in less than 1% of the
patients were itching, candidiasis, fatigue, malaise, headache, chest pain, flatulence, abdominal distension,
glossitis, urine retention, dysuria, oedema, facial swelling, erythema, chills, tightness in the throat,
substernal pain, epistaxis, and mucosal bleeding.
Laboratory changes most commonly reported involved elevated hepatic enzymes (AST, ALT, alkaline phosphatase,
lactate dehydrogenase). Haematological abnormalities (decreased haematocrit/haemoglobin, leucopenia,
lymphopenia, thrombocytopenia or increased lymphocytes, monocytes, basophils, eosinophils and platelets),
decreased albumin and total proteins, increased creatinine, and the presence of red blood cells and hyaline
casts in the urine are less frequent.
Limited information is available on the acute toxicity of sulbactam sodium in humans. The molecular weight,
degree of protein binding and pharmacokinetic profile of sulbactam suggest that this compound may be removed by
haemodialysis.
When concomitant therapy with other antibacterials is indicated, SULBACIP I.V. should be reconstituted and
administered separately.
Before Opening
Store below 25°C. Protect from light.
Reconstituted Solution
Reconstituted solution is stable for 7 days at 2-8°C, and for 24 hours at room temperature.
SULBACIP I.V. 1 gm........................ Available in a vial of 20 mL
SULBACIP I.V. 2 gm........................ Available in a vial of 20 mL
- Intrinsic activity against Acinetobacter spp., including carbapenem-resistant isolates
- Activity is mediated by its interaction with PBPs, rather than an inhibitory effect on beta-lactamases
- Alters the permeability of the outer membrane of Acinetobacter spp., resulting in the leakage of
beta-lactamases and, thus, better penetration by other antibacterial agents.
- Gives the flexibility to combine with any other antibacterial, depending on the susceptibility report.
- Results in 16 and 8- fold reductions in carbapenem and colistin MIC values against Acinetobacter.
- Improved survival rate observed in pneumonia model when used in combination with meropenem.
- Recent literature recommends the use of minimum of 6 gm/day up to maximum 12 gm/day of sulbactam.
- Sulbactam when used at high dose in pneumonia model, reaching a t>MIC similar to that of imipenem (1.84
versus 2.01 hours), was as efficacious as imipenem.
- Effective even against carbapenem resistant Acinetobacter bacteraemia, VAP, meningitis.
- No major adverse effects were observed at doses of 6-12 gm/day with sulbactam.
1. J Antimicrobial Chemother 2009; 63:243-245
2. Rev Infect Dis 1986; 8:S496-S502
3. Future Microbiol 2008; 3:649-660
4. J Antimicrob Chemother 2011; 66:1625-1630
5 Indian J Med Microbiol 2005; 23:189-191
6. J Assoc Physicians India 2010; 58:2531
7. Indian J Med Microbiol 2011; 29:269-274
8. Indian J Crit Care Med 2011; 15:96-101
9. J Med Assoc Thai 2010; 93(2):161-171
10. Clin Microbiol Infect 2002; 8:144-153
11. Antimicrob Agents Chemother 1999; 43:1406-1411
12. J Antimicrob Chemother 1990; 26:677-682
13. Int J Antimicrob Agents 2011; 37:102-109
14. Product Info Unasyn (Ampicillin/Sulbactam)
15. Semin Respir Crit Care Med 2007; 28:662-671
16. J Antimicrob Chemother 2001; 47:479-482
17. J Antimicrob Chemother 1996; 37:155-160
18. Int J Antimicrob Agents 2006; 28:454-456
19. Clin Microbiol Infect 2004; 10:1089-1104
20. Int J Antimicrob Agents 2012; 39:177-185
21. Int J Antimicrob Agents 2002; 20:390-392
22. Antimicrob Agents Chemother 2000; 44:1035-1040
23. J Infect 2008; 56:432-436
24. J Antimicrob Chemother 1998; 42:793-802
25. Scan J Infect Dis 2007; 39:38-43
26. J Antimicrob Chemother 2004; 53:393-395
27. Pharmacother 2007; 27:1506-1511
28. Jpn J Infect Dis 2009; 62:461-463
29. J Hosp Infect 2003; 54:32-38
30. Clin Infect Dis 1996; 22:1026-1032
31. Clin Infect Dis 2002; 34:1425-1430
32. Clin Infect Dis 1997; 24:932-935
33. Microb Drug Resist 2008; 14:233-237
34. Pharmacother 2002; 22:527-532
35. Microb Drug Resist 1995; 1:249-253
36. Int J Antimicrob Agents 2003; 21:58-62