Introduction
Inhaled therapy is the mainstay of asthma management, and pressurized metered dose inhalers (pMDIs) are the most commonly used devices for delivering inhaled asthma medications. pMDIs are portable, convenient and multi-dose and these advantages have made them very popular with patients. Despite possessing a number of advantages, the pMDI also suffers from some inherent limitations.
Hand Lung Coordination
It has been reported that nearly 80% of patients fail to coordinate the actuation of the spray with the inhalation, thus reducing the drug delivery to the lungs.1
Cold Freon Effect
This is an additional handling problem which may cause some patients (especially young children) to stop inhaling when the propellant spray hits the back of the mouth.
Oropharyngeal Deposition
The spray released from the pMDI comprises of large rapidly moving propellant droplets, which readily impact in the mouth and pharynx, so that only a small fraction of the dose deposits in the lungs. Nearly 80% deposits in the oropharynx and 10-20% in lungs.
Spacer devices help to overcome these inherent drawbacks of the pMDIs. The main function of these devices is to act as a reservoir where the actuated aerosol cloud can be held prior to inhalation by the patient, thus reducing the need for hand-lung coordination.
Working of a Spacer
Spacers extend the mouthpiece of the inhaler and direct the cloud of medication towards the mouth. When the pMDI is fired or actuated, the aerosol cloud produced from the spacer device is finer and slower moving than when released from a pMDI alone. This results in evaporation of the propellants and settling down of the larger particles in the spacer. This reduces the amount of particles in the mouth and throat, decreasing the amount of drug absorbed systemically and also increases the amount of drug reaching the lower airways.2
Advantages/Need of Spacer Devices
Making pMDIs Easier to Use
Nearly 80% of the patients fail to use the pMDI correctly, with the major problem being co-ordinating the actuation with inhalation.1 This is easily overcome by a spacer, which hold the medication for a short time till the patient inhales.
Reducing Oropharyngeal Deposition
Normally, with a pMDI, close to 70-80% of the drug is deposited in the oro-pharynx. This is due to the speed with which the drug is emitted. When a spacer is used alongwith a pMDI, oropharyngeal deposition drops by more than 50% since most of the larger particles settle on the walls of the spacer. This reduces the likelihood of local as well as systemic side effects.3
Improving Pulmonary Deposition
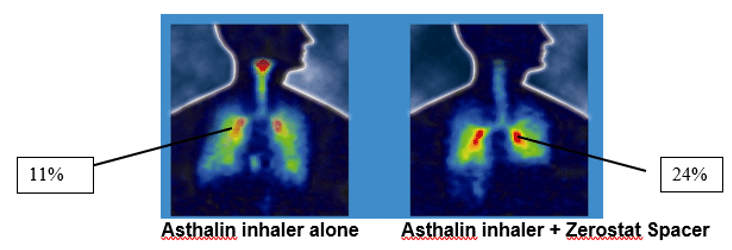
The velocity of the aerosol spray is reduced as it passes through the spacer. Also, the propellant droplet size is reduced by evaporation. This results in increased lung deposition. For, example a study conducted showed that the mean percentage of salbutamol deposited in the lungs was found to be 11% with the pMDI alone, but the deposition increased to almost 24% with the use of a Zerostat spacer.
The First Gamma Scintigraphy Study in India
The lung deposition of inhaled salbutamol was assessed using Asthalin inhaler with Zerostat Spacer using Gamma scintigraphy. The test drug (salbutamol) was radiolabelled using the radionucleide Technetium. Immediately after inhaling the radiolabelled aerosol, the subject had the imaging performed using a gamma camera. The mean percentage of salbutamol deposited in lungs was found to be 11% with the MDI alone. This was seen to increase to almost 24% with the addition of Zerostat Spacer. Also the Zerostat Spacer reduced the oropharyngeal deposition of the drug as can be seen from the figure.4
As an Alternative to Nebulizers in Acute Asthma Attacks
A systematic review of studies of Treatment in children with acute exacerbations of asthma has found a pMDI with a spacer to be as effective as a nebulizer. 5
Also, Compared to the Nebulizers, pMDIs with Spacers have the Following Benefits
- Cause fewer side effects due to less drug dose used
- Provide greater savings in cost
- Require no electric power
- In case of the patient being hospitalized, facilitates faster discharge from the emergency department
Who Should Use Spacers?
- Children and the elderly
- Patients with co-ordination problems
- Those who are prescribed high-dose inhaled steroids (more than 1,000 mcg/day).
- Patients who are prescribed anti-cholinergic (to avoid the spray particles from reaching the eyes).
- Patients with acute asthma requiring high-dose bronchodilators, as a substitute to nebulizers.
- Infants and small children who need inhaled drugs, as spacers can be used with masks
Factors which Affect the Dose Delivery through the Spacer Device - Spacer Design
Chamber Size
The cumbersome and bulky nature of the spacer has always been an impediment for its usage by patients. Hence, there is a need to balance the dimensions of spacer for optimal aerosol delivery and clinical convenience. As the spacers have evolved there has been a shift from the large volume static spacers to small volume anti static spacers, which are portable and easy to use.
There has always been a debate whether the large volume or the small volume spacers are ideal. Evidence supporting the effect of spacer size on clinical evidence is sparse. In one of the earlier studies supporting the development of more patient-friendly, small volume valved holding chamber; Corr et al evaluated the fine particle output through spacers of varying sizes. Results of the study indicated that a tube 11 cm in length and 3.5 cm in diameter, delivered majority of respirable aerosol, measured using a cascade impactor. Any further increase in these dimensions failed to confer any significant benefit in terms of respirable dose. 6
Factors such as ease of use, pocketability, portability, patient satisfaction should be considered important while selecting spacers, as they will help in improving adherence to usage of spacers. There is no benefit of developing a spacer which shows a high in vitro performance in terms of respirable fraction if the patient is not satisfied with it and does not use it.
“Engineers are likely to opt for the most efficient device in terms of aerosol output, clinicians have to take other factors into account viz; portability, ease of use to obtain optimum patient compliance.”
J Aerosol Med Pulm Drug Deliv 2014; 27(Suppl 1): S4-S23
Chamber Shape
Spacers are generally of a cone, pear or tube (cylinder) shape. The closer the shape of the spacer to the shape of the aerosol plume, the better it is. The wider the spacer lesser is the drug loss in the spacer. Pear-shaped devices may be particularly effective in reducing, particle loss within the device and the mouth given their ability to collect the aerosol plume and allow the propellant to evaporate.7 A cylindrical spacer will cause more aerosol deposition onto the walls of the spacer, leading to reduced delivery of the drug dose to the patient. Therefore, the ideal shape for a spacer is a cone or a pear shape.
Electrostatic Charge
Static charge plays an important role in the performance of the spacer. The surface of plastic spacer accumulates static charge. Highly charged aerosol particles from an MDI are rapidly deposited on the walls of the static spacer, rendering them unavailable for inhalation. This leads to a significant reduction in dose available for inhalation thus decreasing the delivery of drug to the lungs.
This electrostatic charge varies in a random manner, depending upon the condition of the spacer – plastic spacers initially have a strong electrostatic charge, which encourages the deposition of aerosol particles on the inside walls of the spacer.3 However, after repeated use, the deposition of drug on the surface of the spacer has an antistatic effect. Thus the dose available to the patient is likely to be strongly influenced by the condition of the spacer, which will vary with time. Re-use of the spacer will initially be associated with increasing drug availability, but the situation is complicated by the regular cleaning of the spacer device, which is advised to the patients. This is likely to reduce the subsequent availability of drug from the spacer, by permitting the further deposition of aerosol particles on its newly cleaned walls. This could result in a cycle of greater drug availability, despite apparently consistent dosing. This is of major concern in children in whom drug delivery is already highly variable due to age – specific breathing patterns such as low tidal volume and inspiratory flow rates.7
Effect of Electrostatic Charge on Drug Delivery through Various Spacer
Deposition pattern of radio-aerosol after inhalation through a static and a non-static (detergent-coated) spacer devices.
The influence of static charge on drug deposition in the lungs was evaluated using a detergent-coated (non-static) and a non-coated plastic spacer (static). Lung deposition of radiolabeled salbutamol was assessed in healthy adults, using imaging techniques.
There was a three-fold increase in lung deposition from non-static spacers compared to static spacers (45.6% Vs 11.5%). The mean amount of salbutamol remaining in the static spacers was 76.7% compared to 33.1% in the static spacers.8
Barry and O’Callaghan found that coating the inside of a plastic spacer with an anti-static lining, increased the fine-particle (<5 µm) mass of pMDI budesonide from 30.5 + 8.8 µg/actuation (untreated device) to 69.3 + 17.9 µg/actuation.9
Features of Mini Zerostat Spacer
Mini Zerostat
Diameter – 62 mm
Height – 114 mm
- Attractive design and is about 30% more compact
- Easy to carry
- Performance better as compared to international cylindrical anti-static VHC
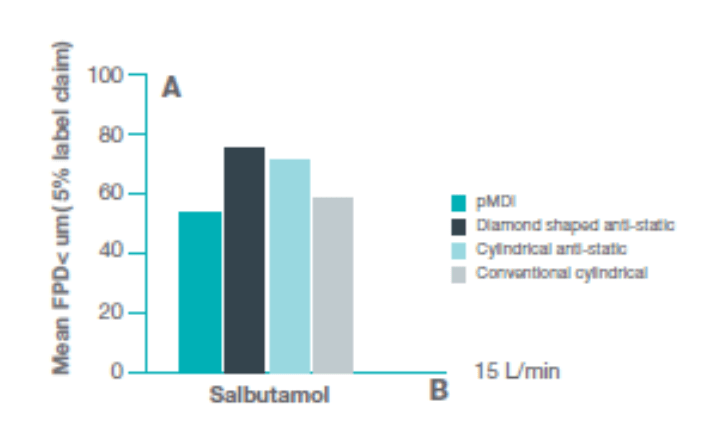
A study compared the performance of diamond shaped, anti-static, 140 ml VHC with an anti-static cylindrical VHC and a conventional cylindrical VHC.
At 15 Lpm the mean FPD* <5 µm (% label claim) was significantly higher (p<0.0001) with the diamond shaped anti-static VHC and the cylindrical anti-static VHC compared with the conventional cylindrical anti-static VHC compared to the pMDI. Between VHC comparisons indicate that a significantly higher dose delivery was achieved with the diamond-shaped anti-static VHC compared with the cylindrical anti-static VHC (p=0.0025). These results indicate that use of anti-static VHC facilitates the output of aerosol with a better aerodynamic particle size distribution compared with that available from a conventional VHC.10
*FPD – Fine Particle Dose
Performance of Mini Zerostat Spacer
Comparative Data: By Cascade Impacter
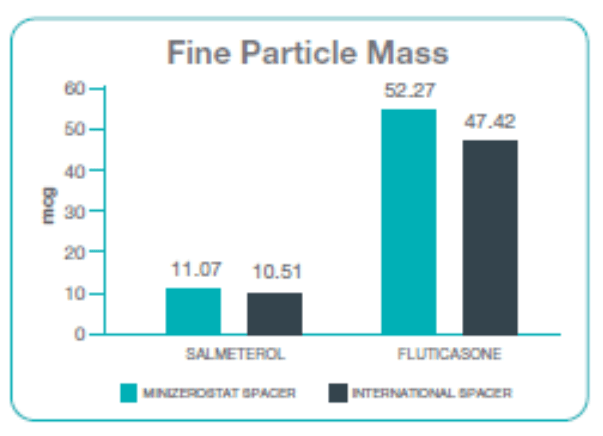
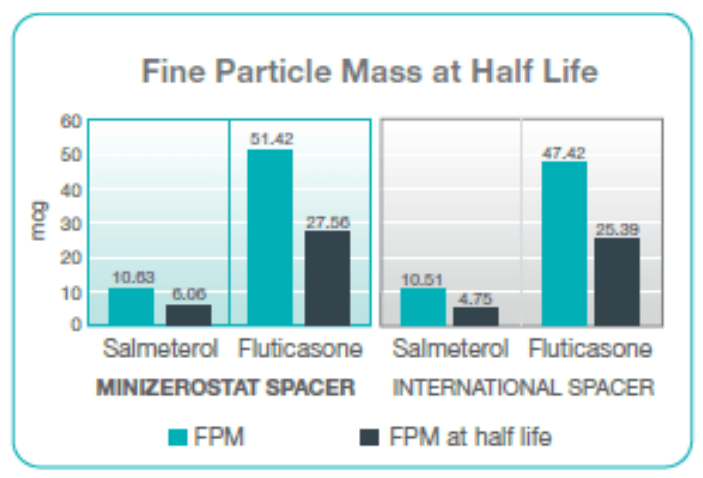
Fine Particle Mass
Performance of Minizerostat spacer was seen to be comparable with marginal higher FPM as compared to the international spacer.
Comparison in terms of half-life
Half-life of an aerosolized drug in a spacer is defined as the time taken for the FPD to fall to 50% from its zero or no delay value, when delays between the pMDI actuation and the onset of inhalation are introduced.
The half-life of the Seroflo 125 inhaler using Minizerostat spacer is approximately 45 seconds, that is, the aerosol particles stay in suspension and are available for inhalation up to as long as 45 seconds to the patient. This makes the Minizerostat easier to use for children, as well as adults, since it requires less technique dependent co-ordination and hence, has more advantages over international spacer.
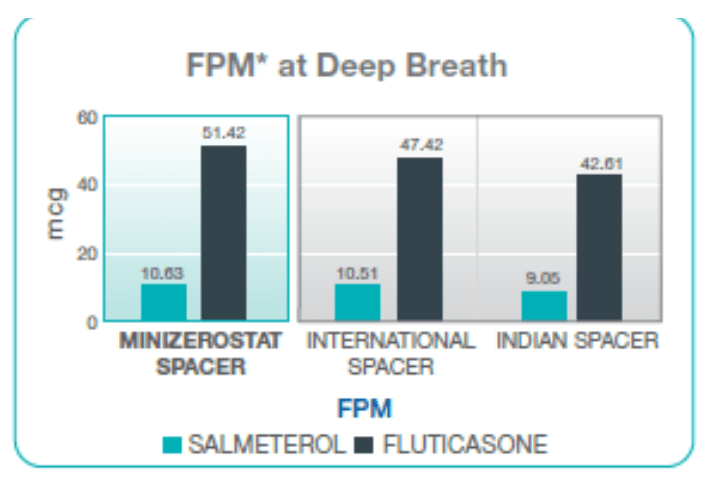
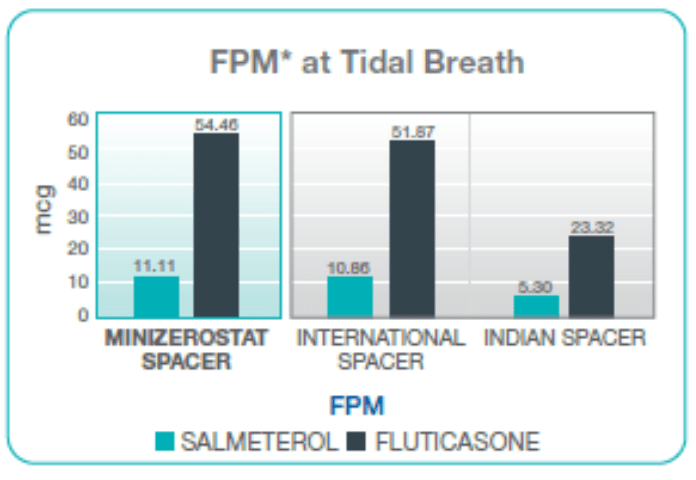
Fine Particle Mass with Single Breath vs Multiple Breaths
Instructions for Use
Step 1
Remove the mouthpiece cap from the mouthpiece of the inhaler. Shake the inhaler well.
Step 2
Insert the inhaler firmly into the opposite end of the Minizerostat spacer.
Step 3
Breathe out fully. Remove the mouthpiece cap from the Minizerostat spacer and place the mouthpiece in your mouth. Close your lips firmly around the mouthpiece to create a good seal as shown in the picture (do not bite it).
Step 4
Press down the canister of the inhaler to release a dose of medicine into the Mini Zerostat spacer. You will see the dose released in the spacer.
Step 5
Breathe in slowly and deeply through your mouth, thus inhaling the medicine through the Minizerostat spacer. Remove the spacer from your mouth and hold your breath for about 10 seconds, or as long as is comfortable. Breathe out slowly.
Or
Keep your lips firmly around the mouthpiece and continue to breathe normally three to five times through the Minizerostat Spacer. Administer one dose at a time. If a second dose is required, wait for a minute and then repeat steps 1 to 5.
For Children
Children should use the Minizerostat spacer under adult guidance. For children below 4 years of age, a face mask (Baby Mask/Infant Mask) attached to the Minizerostat spacer should be used to facilitate administration.
References
- Am J Health-Syst Pharm 2001; 58:585
- World J Pharm Sci 2015; 3(7): 1387-1396
- Clin Pharmacokinet 2004; 43 (6): 349-360
- Data on file, Cipla Ltd.
- The Cochrane Database of Systematic Reviews 2003, Issue 2. DOI: 10.1002/14651858.CD000052.
- J Aerosol Sci 1982; 13:1-7.
- J Aerosol Med Pulm Drug Deliv 2014; 27(Suppl 1): S4-S23.
- Swiss Med Wkly 2001;131:14–18.
- Eur Resp J; 1997, 10: 1345-48.
- Br J Clin Pharmacol 1995; 40(1):76-78.
- J Aerosol Med Pulm Drug Deliv 2014; 27 (Suppl 1): S24-S36
- Data on file 2017, Cipla Ltd.