Introduction to ACS
As per World Health Organisation (WHO) data, the prevalence of coronary artery disease (CAD) continues to rise in India with rapid ‘epidemiological transition’. It has already surpassed communicable diseases as the major cause of mortality in India. It has been projected that in India, between 1990 and 2020, there will be 117% and 105% rise in mortality from CAD in men and women respectively. Coronary artery disease can lead to acute coronary syndrome (ACS), a condition characterized by signs and symptoms of sudden myocardial ischemia. Acute coronary syndrome includes unstable angina, non-ST elevation myocardial infarction (NSTEMI) and ST-elevation myocardial infarction (STEMI). It is responsible for one-third of total deaths in people >35 years.1,2,3
Myocardial infarction occurs when one of the coronary arteries in the heart is blocked suddenly or has extremely slow blood flow. The usual cause of sudden blockage in a coronary artery is the formation of a blood clot (thrombus) that typically forms inside the artery that already has been narrowed by CAD. Myocardial infarction can be fatal, but treatment has improved dramatically over the years.4,5
Pathophysiology
Unstable angina and NSTEMI are usually caused by the common pathophysiological mechanism of the unstable atherosclerotic plaque that results in the formation of either a nonocclusive thrombus or complete thrombosis of a vessel supplying a well collateralised area.6 ST-elevation myocardial infarction is most often caused by complete and persistent blockage of a coronary artery by thrombus. The abrupt disruption of blood flow is due to plaque rupture, erosion, fissuring or dissection that results in an obstructing thrombus.7,8 Myocardial damage begins as soon as the coronary blood supply is blocked, longer the blood supply is blocked greater the amount of cardiac muscle lost.8 In the patients surviving STEMI, the infarcted muscle is gradually replaced by fibrosis (scar tissue) and the extent of damage will determine the overall pumping ability of the heart and is a determinant of ‘heart failure’ and longer term survival.8
The major risk factors for STEMI are dyslipidemia, diabetes, hypertension, smoking and family history of CAD.7
Epidemiology of ACS
According to the Global Burden of Disease study, age-standardized CVD death rate is higher among Indian population compared to global average population, 272 per 100000 vs. 235 per 100000 population, respectively. Indian population shows higher incidence of CAD compared to western population, in 2004, World Health Organisation (WHO) reported CVD death rates for all ages as 174.7 per 100,000 in Britain, 178.8 per 100,000 in US, 279.5 per 100,000 in China and 381.5 per 100,000 in India.2,9 According to the WHO data, the prevalence of CAD continues to rise in India with rapid ‘epidemiological transition’. About 30 million individuals in India have CAD. Prevalence of CAD among young population in Asian Indians is greater than Western countries, 11–16% vs. 2–5%, respectively.
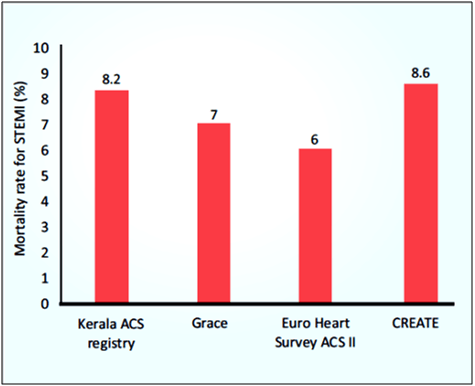
Nearly 3 million STEMI are estimated to occur in India per year. Patients with ACS in India have a higher proportion of STEMI as compared to developed countries. According to a study in patients with MI, young Indians had 10 times higher risk for developing MI compared to white population. The registries from different regions of India including Himachal Pradesh from North, Assam from North East (NE), Kerala and Chennai from South and multicity, multi- hospital CREATE Registry, reported that patients with ACS in India have a higher proportion of STEMI as compared to developed countries.2,10 In-hospital mortality rate for STEMI was higher in the Kerala ACS Registry (8.2%) compared to GRACE (7%) and Euro Heart Survey ACS II (6%), but similar to CREATE (8.6%), which included mortality over 30 days (see Fig. 1).2
Symptoms
Substernal chest pain (angina) is the classic symptom of ACS that is described as crushing or pressure-like feeling in the chest that radiates to jaw and/or left arm. In addition, the other chief complaints include difficulty breathing, lightheadedness, isolated jaw or left arm pain, nausea, epigastric pain, diaphoresis and weakness.2 However, series of STEMI from India report that more than 90% of patients (91–99%) present with classical chest pain, and commonly associated with sweating (70–80%).2
Diagnosis of STEMI
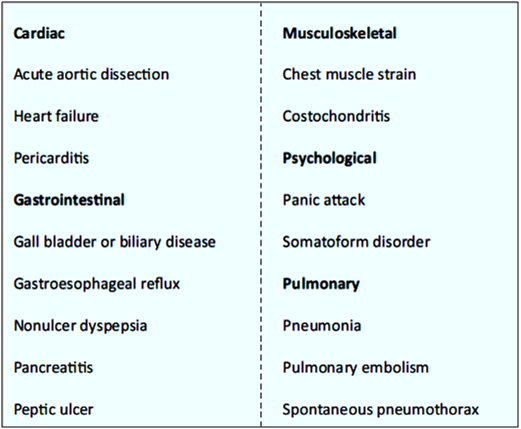
The patient’s clinical history, presenting symptoms, electrocardiographic results and biomarker levels are all evaluated. Conditions other than coronary ischemia, with cardiac or noncardiac causes can lead to similar symptoms and should be ruled out (see Table 1).
Only 20–30% of patients presenting with acute chest pain are ultimately confirmed to have ACS upon detailed evaluation (see Fig. 2).2
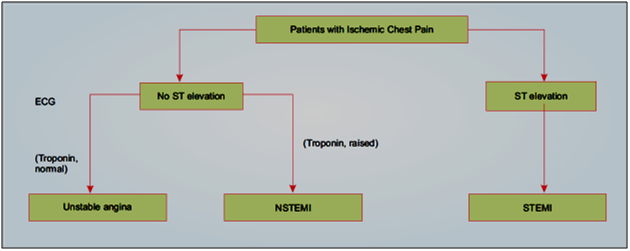
ST segment elevation myocardial infarction is characterized by myocardial ischemia that results in persistent ST segment elevation on electrocardiogram (ECG) and subsequent release of biomarkers of myocardial damage. Increased biomarkers alone in the absence of ST segment elevation constitute NSTEMI. NSTEMI may manifest with transient/persistent ST segment depression and/or T wave inversion in ECG. Prolonged ischemic chest pain without elevation of markers of myocardial necrosis constitutes unstable angina. In India, STEMI is the most common form of ACS that accounts for 40–60% of ACS cases.2
Electrocardiographic Findings
The AHA and the ACC recommend “within 10 minutes of emergency department arrival, a 12-lead electrocardiogram (ECG) be performed in patients with symptoms consistent with ACS and interpreted by an experienced physician”.1, 11 ST segment depression, symmetric T wave inversion and Q waves are associated with an increased risk of MI.
A diagnosis of STEMI that mandates emergency reperfusion requires ST elevation equalling or exceeding the following cut-points, in at least two contiguous leads (using the standardization of 1.0 mV = 10 mm). Criteria to diagnose STEMI include ST segment elevation of11,12,13
- ≥2.5 mm in men <40 years, ≥2 mm in men and ≥1.5 mm in women for leads V2 and V3
- ≥1 mm for leads V1, V4-6, I, II, III, aVL and aVF
- 0.5 mm for leads V3R and V4R (right-sided leads) and V7-9 (posterior leads)
- In patients with inferior MI, it is recommended to record right precordial leads (V3R and V4R) seeking ST-segment elevation, to identify concomitant right ventricular (RV) infarction.
- ST-segment depression in leads V1–V3 suggests myocardial ischaemia, especially when the terminal T-wave is positive (ST-segment elevation equivalent), and confirmation by concomitant ST-segment elevation ≥0.5 mm recorded in leads V7–V9 should be considered as a means to identify posterior MI.
- The presence of a Q-wave on the ECG should not necessarily change the reperfusion strategy decision.
Cardiac Biomarkers
Cardiac biomarkers help in determining whether the patient is having or has recently had an acute MI (either an NSTEMI or a STEMI). Troponins I and T levels increase within 4-6 hours of myocardial injury; troponin I levels remain increased for 4-7 days, and troponin T levels remain increased for 10-14 days. Myocardial necrosis is diagnosed when a single troponin elevation greater than the 99th percentile of an agreed-upon reference control group is required. Cardiac troponins are the preferred biomarkers for diagnosing acute MI because elevated levels correlate with a more accurate diagnosis, predict a high risk of future cardiac events even when levels of the myocardium-specific biomarker creatine kinase-MB (CK-MB) are normal or only mildly elevated, and elicit fewer false positives when concurrent skeletal muscle injury is present (after trauma or surgery).
In case if a laboratory is not able to process troponins, CK-MB is considered a reasonable alternative. CK-MB is a cardiac-specific enzyme that is released within 4-6 hours of injury and remains elevated for 48-72 hours after injury. Two consecutive levels of CK-MB greater than the 99th percentile of a reference control group contribute to the diagnosis of acute MI.1,11
Impact and Prognosis
Complications in patients post-MI are well documented and include a significant risk of additional cardiovascular events such as heart failure, angina, arrhythmias, stroke or death. With the use of reperfusion and preventative therapies, short-term mortality following MI has decreased.14
Management of STEMI
Reperfusion therapy should be started as soon as possible and preferably within 90 min from first medical contact (FMC). However, the total ischemic time (time between symptom onset and reperfusion therapy) is the most important factor to achieve the best possible outcome for the patient. Whatever the choice of reperfusion therapy, patient decision time to seek medical help is crucial. In India various registries have shown trends of late presentation. Shortening the time from symptom to reperfusion and choosing the optimal reperfusion strategy for STEMI patients are great challenges in practice.2
Acute Management of STEMI
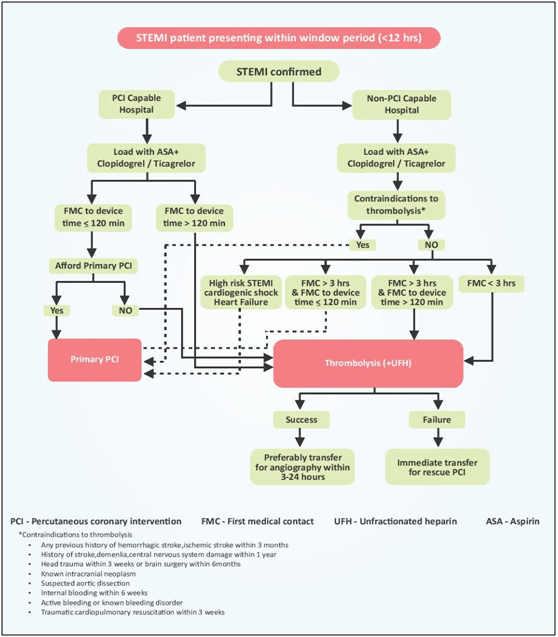
In all patients with suspected STEMI, a 12-lead ECG must be acquired and interpreted as soon as possible at the time of first medical contact (FMC) to facilitate early STEMI diagnosis and triage. When STEMI diagnosis is made in the pre-hospital setting, immediate activation of the catheterization laboratory not only reduces treatment delays but may also reduce patient mortality.11 A delay by the patient and FMC physicians often contributes to patient presenting out of the window period (12 h after the chest pain). Figure 3 represents an algorithm to manage patients presenting within window period (12 h). A cut-off of 12 h is considered because maximum benefit of revascularization strategy occurs during this time period and after this time period benefit of revascularization in uncomplicated STEMI is low. Thrombolysis with the tissue plasminogen inhibitor (tenecteplase or alternately reteplase) is the preferred fibrinolytic agent and is the best option in case patient presents to a non-PCI center.15
Fibrinolysis Therapy and Pharmaco-Invasive Strategy
Timely reperfusion results in better myocardial salvage and preservation of left ventricular function. According to many guidelines, primary PCI is the preferred mode of reperfusion but only few patients with STEMI can avail this form of reperfusion within recommended timelines. Thrombolysis with fibrinolytic therapy is an important reperfusion strategy in settings where primary PCI cannot be offered in a timely manner (see Table 2).11 Pharmacoinvasive therapy wherein initial timely thrombolysis followed by early PCI is an attractive option of reperfusion in STEMI and may bridge the gaps in systems of care. Thrombolysis prevents approximately 30 early deaths per 1000 patients treated within 6 h after symptom onset. It is currently the most practiced form of reperfusion method in India. It can be initiated by a physician in an emergency department when there is no plan to transfer the patient to a PCI capable centre for primary PCI. Failed thrombolysis can be diagnosed by persisting or worsening chest pain or less than 50% resolution of ST-segment elevation after 90 min of thrombolysis in the lead showing maximum ST-segment elevation at presentation.
Rescue PCI is advocated for such patients and patients should be transferred to a PCI-capable centre immediately.2
Pharmaco-invasive strategy consists of early thrombolysis followed by either rescue PCI for patients with failed thrombolysis, or non-urgent coronary angiography to determine the need for additional revascularization within 3–24 h. PCI performed 3 h after thrombolysis precludes the early pro-thrombotic phase and reduces the chances of re-occlusion. Furthermore, this delay may also be the reason for decrease in bleeding complications that were seen with facilitated approach. Hence, PI strategy is appropriate for patients with STEMI who are eligible for treatment with thrombolytic drugs and in whom FMC to balloon time is >120 min. Current STEMI guidelines from the American College of Cardiology/American Heart Association (ACC/AHA) and the European Society of Cardiology (ESC) also recommended that patients going to a non-PCI-capable hospital should receive fibrinolysis immediately, if the expected FMC to device time is >120 min, and then be transferred to a PCI capable hospital within 24 h for coronary angiogram and if needed PCI.9,15
Secondary Prevention Strategy Post MI
According to the 2013 National Institute for Health and Care Excellence (NICE) guidelines, the secondary prevention strategies include cardiac rehabilitation (initiated as soon as possible after admission and before discharge from hospital), recommend mediterranean-style diet (more bread, fruits, vegetables and fish; less meat and replace butter and cheese with products based on plant oils), advise to limit their alcohol consumption and quit smoking, regular physical activity for 20-30 minutes a day, to maintain a healthy weight and to adhere to their treatment plan.15
Tenecteplase: A Fibrinolytic Agent
Mechanism of Action
Tenecteplase is a modified form of human tissue plasminogen activator (tPA) that binds to fibrin and converts plasminogen to plasmin. In the presence of fibrin, Tenecteplase conversion of plasminogen to plasmin is increased relative to its conversion in the absence of fibrin. This fibrin specificity decreases systemic activation of plasminogen and the resulting degradation of circulating fibrinogen as compared to a molecule lacking this property.16
Formulation and Dosage
Tenecteplase is available as vials containing lyophilized product in the strengths of 30 mg (6000 IU), 40 mg (8000 IU) and 50 mg (10,000 IU) respectively.16
Therapeutic Indication
Tenecteplase is indicated in adults for thrombolytic treatment of suspected myocardial infarction with persistent ST segment elevation or recent left Bundle Branch Block within 6 hours after the onset of acute myocardial infarction (AMI) symptoms. Treatment should be initiated as soon as possible after the onset of AMI symptoms.16
Clinical Evidence on Tenecteplase
Tenecteplase Dose Finding
Thrombolysis in Myocardial Infarction (TIMI) 10B: Tenecteplase compared with Front-Loaded Alteplase in AMI17
Background and Aim
Bolus thrombolytic therapy is a simplified means of administering thrombolysis that facilitates rapid time to treatment. TNK-tissue plasminogen activator (TNK-tPA) is a highly fibrin-specific single-bolus thrombolytic agent. The TIMI 10B trial compared prospectively the angiographic efficacy and safety of several doses of TNK-tPA (tenecteplase) and tPA (alteplase) to identify an appropriate dose for testing in a large trial.17
Design and Methods
- A total of 886 patients with acute STEMI presenting within 12 hours were randomized to receive either a single bolus of 30 or 50 mg tenecteplase or front-loaded alteplase and underwent immediate coronary angiography.
- The 50 mg dose was discontinued early because of increased intracranial hemorrhage and was replaced by a 40 mg dose.
- Two cohorts were prespecified for analyses: The safety cohort comprised patients who received any amount of study drug (856 patients), and the “efficacy-evaluable” cohort comprised patients who received study drug and had an evaluable 90-minute angiogram (837 patients).
Pharmacokinetics
- Among the 159 patients with pharmacokinetic samples, the plasma clearance of tenecteplase ranged from 98.4±42 to 119.0±49 mL/min across the 30-, 40- and 50-mg doses, compared with 453±170 mL/min for alteplase.
- The corresponding plasma elimination half-life of tenecteplase ranged from 5.5±5.5 to 21.5±8.2 minutes. The corresponding half-life for alteplase is 3.5±1.4 minutes.
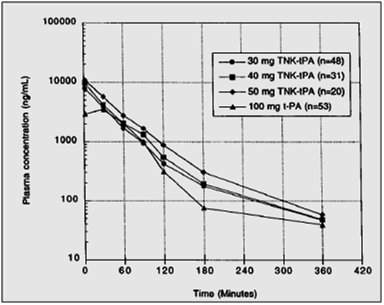
- Figure 4 shows the TNK-tPA plasma levels over time for the 3 doses of tenecteplase and alteplase. As shown, after a single bolus of tenecteplase, the plasma concentration was initially higher, but the area under the curve approximates that of alteplase given as a bolus and 90- minute infusion.
Mean plasma concentration vs. time data for tenecteplase 30, 40, and 50 mg bolus and alteplase recombinant alteplase 100 mg as accelerated infusion regimen
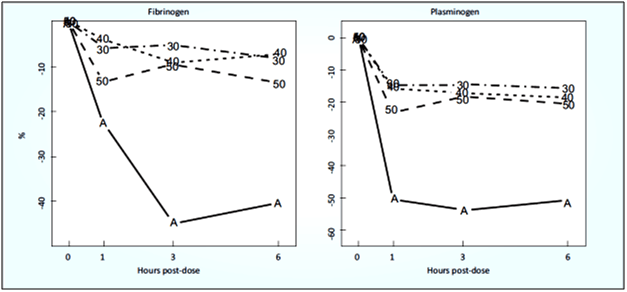
High Fibrin Specificity, Low Plasminogen Specificity
Tenecteplase has a far less effect on systemic coagulation factors than alteplase, because it is more fibrin specific (see Fig. 5). There was a 5-10% drop in fibrinogen over the first six hours after administration of tenecteplase, compared with a 40% drop after alteplase. The fall of plasminogen was only 10-15% after tenecteplase, compared to a 50% drop after alteplase.
Efficacy and Safety of Tenecteplase
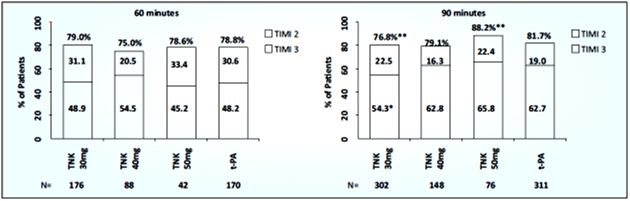
- Tenecteplase 40 mg and alteplase produced similar rates of TIMI grade 3 flow at 90 minutes after the start of thrombolysis, 62.8% vs. 62.7%, respectively (see Fig. 6).
- The 30-mg dose of tenecteplase had a significantly lower rate of TIMI grade 3 flow than alteplase (54.3%, p=0.035), whereas the 50-mg dose achieved 65.8% TIMI grade 3 flow. At 60 minutes, there were no significant differences in the rates of TIMI grade 3 flow or patency (see Fig. 6).
- A prespecified analysis of weight-based tenecteplase dosing using median TIMI frame count demonstrated a dose response (p=0.001).
- Similar dose responses were observed for serious bleeding and intracranial hemorrhage, but significantly lower rates were observed for both tenecteplase and alteplase after the heparin doses were lowered and titration of the heparin was started at 6 hours.
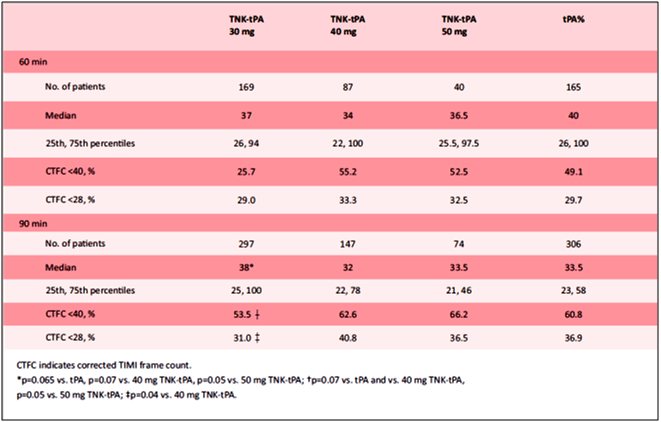
- Table 3 depicts the TIMI frame count results by treatment group. The median TIMI frame count at 60 minutes was slightly, but not significantly, lower for tenecteplase 40 mg than for alteplase (34 vs. 40 frames, p=0.33).
Conclusion
TNK-tPA is a promising single-bolus thrombolytic agent. At a 40-mg dose, the rate of TIMI grade 3 flow was comparable to that with the 90-minute regimen of tPA. Weight-adjusting TNK-tPA appears to be important in achieving optimal reperfusion; reduced heparin dosing appears to improve safety for both agents.
ASSENT-2 Double-Blind Randomised Trial: Ease of Administration of Tenecteplase Facilitate More Rapid Treatment18
Aim
A double-blind, randomized, controlled trial was conducted to evaluate the efficacy and safety of tenecteplase compared with alteplase.
Design and Methods
- A total of 6,949 patients with AMI of <6 h duration were randomly assigned to rapid infusion of alteplase (≤100 mg) or single-bolus injection of tenecteplase (30-50 mg according to body weight).
- All patients received aspirin and heparin (target activated partial thromboplastin time 50-75 s).
- Main outcome of the study was equivalence in all-cause mortality at 30 days.
Results
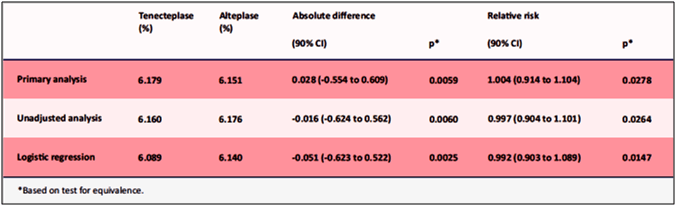
- Tenecteplase showed similar covariate-adjusted 30-day mortality rates compared to alteplase, 6.18% vs. 6.15% (see Table 4). The 95% one-sided upper boundaries of the absolute and relative differences in 30-day mortality were 0.61% and 10.00%, respectively.
- Tenecteplase was well-tolerated with less non-cerebral bleeding complications (26.43 vs. 28.95%, p=0.0003) and less need for blood transfusion when compared to alteplase.
- Similar rate of intracranial haemorrhage, rate of death or non-fatal stroke at 30 days were observed with tenecteplase compared to alteplase, 7.11% vs. 7.04%, respectively.
Conclusion
Tenecteplase and alteplase were equivalent for 30-day mortality. The ease of administration of tenecteplase may facilitate more rapid treatment in and out of hospital.
Tenecteplase in Pharmacoinvasive Therapy
The Strategic Reperfusion Early After Myocardial Infarction (STREAM) Study19,20
Background and Aim
Primary PCI has emerged as the preferred therapy for acute STEMI. Fibrinolysis is a well-accepted alternative, especially in patients presenting early after symptom onset. The STREAM study is an open-label, prospective, randomized, parallel, comparative, international multicenter trial that provided novel information on whether prompt fibrinolysis at first medical contact, followed by timely catheterization or rescue coronary intervention in STEMI patients presenting within 3 hours of symptom onset, represents an appropriate alternative strategy to primary PCI.
Design and Methods
- A total of 1892 patients presenting within 3 hours of symptom onset who demonstrate acute STEMI and in whom primary PCI is not feasible within 60 minutes of a qualifying diagnostic ECG (first medical contact) were included.
- Patients were randomized to fibrinolysis with tenecteplase combined with enoxaparin, clopidogrel and aspirin, and cardiac catheterization within 6 to 24 hours or rescue coronary intervention if reperfusion fails within 90 minutes of fibrinolysis vs. PCI performed according to local guidelines.
- Composite efficacy end points at 30 days were death, shock, heart failure and reinfarction. Safety end points were ischemic stroke, intracranial hemorrhage, and major non-intracranial bleeding. Follow-up is extended to 1 year and includes all-cause mortality.
Efficacy of Tenecteplase
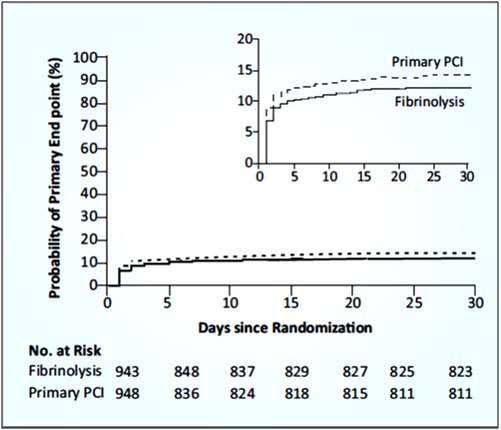
- The primary end point (death from any cause, shock, congestive heart failure, or reinfarction up to 30 days) occurred in 12.4% patients in the fibrinolysis group and 14.3% patients in the primary PCI group (see Fig. 7).
- Tenecteplase showed lower rate of occurrence of cardiogenic shock and congestive heart failure compared to primary PCI group.
- Significantly more open vessels were found on first angiography before PCI in the fibrinolysis group than in the primary PCI group (see Table 5).
- Significantly more bypass surgeries and fewer PCIs were performed in the fibrinolysis group than in the PCI group.
- Rates of stroke were low in the two study groups. There were no cases of intracranial hemorrhage with the dose reduction of tenecteplase in patients ≥75 years of age (0 of 97 patients).
Conclusion
Prehospital fibrinolysis with tenecteplase with timely coronary angiography resulted in effective reperfusion in patients with early STEMI who could not undergo primary PCI within 1 hour after the FMC.
TRANSFER-AMI: Routine Early Angioplasty after Fibrinolysis for Acute Myocardial Infarction21
Background and Aim
The Trial of Routine ANgioplasty and Stenting after Fibrinolysis to Enhance Reperfusion in Acute Myocardial Infarction (TRANSFER-AMI) compared the outcome of a pharmacoinvasive strategy (transfer to a PCI centre for routine early PCI within 6 h) and a standard treatment (early transfer only for failed reperfusion, otherwise catheterisation >24 h) for high-risk STEMI patients receiving thrombolysis at non-PCI centres.
Design and Methods
- A total of 1059 high-risk patients who had a STEMI and who were receiving fibrinolytic therapy at non-PCI centres within 12 h of onset of symptoms were included.
- Patients were randomized to either standard treatment (including rescue PCI, if required, or delayed angiography) or a strategy of immediate transfer to another hospital and PCI within 6 hours after fibrinolysis.
- Patients received aspirin, tenecteplase, and heparin or enoxaparin; concomitant clopidogrel was recommended.
- The primary end point was the composite of death, reinfarction, recurrent ischemia, new or worsening congestive heart failure, or cardiogenic shock within 30 days.
- The secondary endpoints included death or re-infarction at 6 months.
Results
- The 30-day clinical outcomes support the pharmacoinvasive treatment over the standard treatment for high-risk STEMI patients receiving thrombolysis at non-PCI centres.
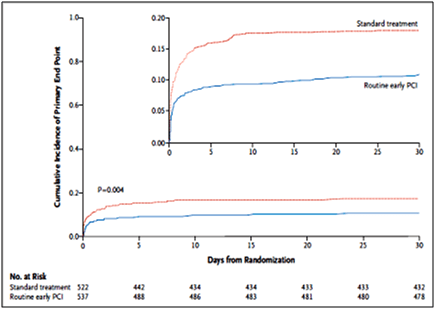
- The pharmacoinvasive treatment group was associated with a significant reduction in the primary endpoint compared to standard treatment, 11.0% vs. 17.2%, respectively (relative risk 0.64; 95% confidence interval 0.47 to 0.87; p=0.004; see Fig. 8).
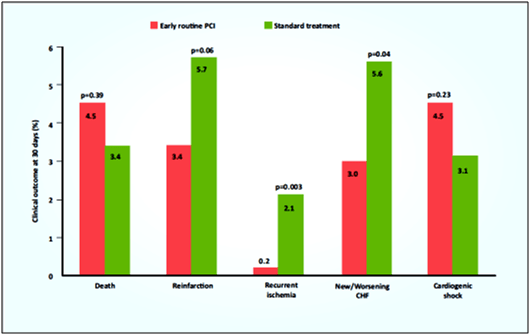
- There were reductions in re-infarction, recurrent ischaemia and new or worsening congestive heart failure (CHF) in the pharmacoinvasive treatment compared to standard treatment group (see Fig. 9).
- There were more mild bleeding events in the pharmacoinvasive treatment compared to standard treatment (13% vs. 9, p=0.04) but no differences in moderate or severe bleeding.
- There were no differences in major or minor bleeding events as assessed by the TIMI criteria. At 6 months, the rates of re-infarction and death did not differ significantly between the two groups.
Conclusion
Among high-risk patients with STEMI treated with fibrinolysis, transfer for PCI within 6 hours after fibrinolysis was associated with significantly fewer ischemic complications than with standard treatment.
STEPP-AMI Study: A Prospective, Observational, Multicenter Study Comparing Tenecteplase-Facilitated PCI versus Primary PCI in Indian Patients with STEMI22,23
Aim
The objective of STEPP – AMI study is to assess the efficacy of pharmacoinvasive strategy in STEMI patients in comparison to primary PCI.
Design and Methods
- This prospective, observational, multicenter pilot study included 200 patients.
- The follow-up period was 30 days, 1-year and 2-year.
- Group A (n=45): Tenecteplase-facilitated PCI + underwent CAG within 3-24 hours with coronary intervention as appropriate.
- Group B (n=155): Primary PCI.
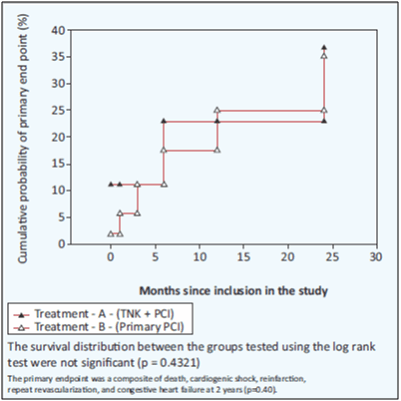
- Primary endpoint was composite of death, cardiogenic shock, reinfarction, repeat revascularization or congestive heart failure at 30 days, up to 1 year and 2 years.
Results
- The infarct-related artery (IRA) patency at angiogram was 82.2% and 22.6% in Group A and Group B, respectively (p<0.001).
- PCI was performed in 73.3% vs. 100% (p<0.001), thrombus was present in 26.7% vs. 63.2% (p<0.001), in Group A and Group B, respectively.
- Significantly more number of patients in Group A had TIMI 3 flow in the culprit vessel at angiogram than Group B, 27.9% vs. 4.5% (p<0.001).
- Failed fibrinolysis occurred in 12.1%.
- Total ischemic time was 245 minutes (185-395) for Group A and 260 minutes (185-390) for Group B. There was no difference in bleeding risk.
- At 30 days follow-up, primary endpoint occurred in 11.1% in Group A and in 3.9% in Group B (p=0.07, RR=2.87; 95% CI: 0.92–8.97 at 30 days and p=0.47, RR=1.31; 95% CI: 0.62–2.76).
- At 1-year follow-up, primary end point occurred in 13.3% and 9% in Group A and Group B, respectively, p=0.40, (RR 0.64; 95% CI 0.24-1.79).
- At 2-year follow-up, primary endpoint occurred in 17.8% in Group A and 13.6% in Group B (p=0.07, RR=2.87; 95% CI: 0.92–8.97 at 30 days and p=0.47, RR=1.31; 95% CI: 0.62–2.76) (see Fig. 10).
- There was no difference in bleeding risk between groups, 2.2% in Group A and 0.6% in Group B (p=0.4).
Conclusion
Fibrinolysis followed by an early coronary angiogram within 3–24 h with PCI, if appropriate, shows similar outcomes when compared to primary PCI in patients with STEMI at 2-year follow-up. These findings suggest pharmacoinvasive strategy to be a viable option in India.
Tenecteplase in Indian patients
Tenecteplase: Effective and Safe in Indian Patients with STEMI24
Aim
A multi-centric, observational, prescription event monitoring study was conducted to assess the safety and effectiveness of tenecteplase in Indian patients presenting with STEMI.
Design and Methods
- Data from 7,668 patients from 1,307 investigator sites across India from January 2011 to February 2016 was collected.
- Of the patients enrolled, majority of the patients were hypertensive (76.71%) followed by 47.97% diabetes patients, 42.01% had dyslipidemia, 24.35% had ischemic heart disease and 40.82% patients were smokers.
Results
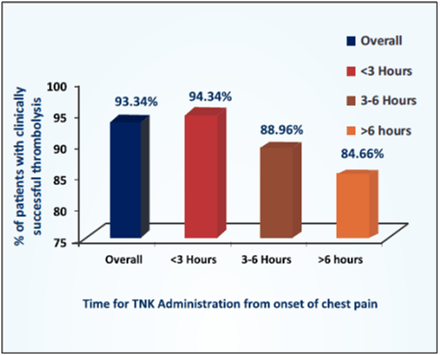
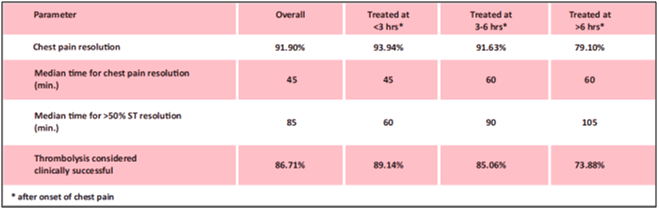
- Tenecteplase achieved thrombolysis in 93.34% of patients. Patients who received tenecteplase within 3 hours of symptoms achieved success rate of 94.34% when compared to lower success rate of 84.66% with delayed administration of tenecteplase (see Fig. 11).
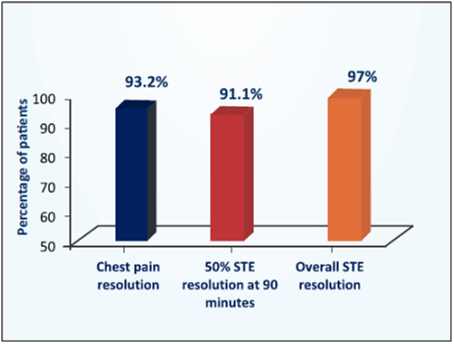
- After pharmacological fibrinolysis, 93.2% patients had chest pain resolution (see Fig. 12). Overall 91.1% patients had 50% resolution of ST elevation at 90 minutes and mean time for 50% ST resolution was 72.06 minutes.
- Mortality was less of about 0.69% before discharge with minor incidence of bleeding excluding stroke (1.77%), any stroke without ICH (0.18%) and any ICH was 0.38%.
Conclusion
Tenecteplase is effective and safe in Indian patients with STEMI including high risk sub-groups. Besides, the study also highlights the importance of early reperfusion therapy.
Tenecteplase: Effective and Safe In-Hospital Use in Indian Elderly Patients with STEMI25
Aim
A post-licensure, observational, prescription event monitoring (PEM) study assessed the safety and effectiveness of tenecteplase in Indian elderly STEMI patients in clinical setting.
Design and Methods
- A total of 2162 patients were enrolled in the study and received weight-adjusted tenecteplase injection.
- Elderly patients were identified, segregated and compared into two groups: elderly (> 60 years) and non-elderly (≤60 years).
- Out of 2162 patients, 805 were elderly patients and 1357 were non-elderly.
Results
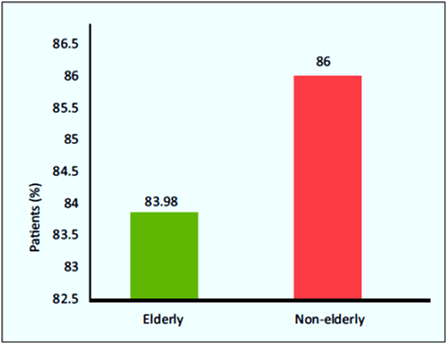
- Tenecteplase showed clinically successful thrombolysis in 83.98% of elderly and 86% of non-elderly group (p=0.22; see Fig. 13).
- The percentage of patients reporting bleeding, stroke, intracranial hemorrhage, myocardial reinfraction, ventricular tachyarrhythmia was similar between the groups with no significant difference.
- Mortality was significantly (p≤0.0001) more in elderly (6.21%) than non-elderly (2.06%) patients.
Conclusion
Tenecteplase shows high efficacy and safety in its in-hospital use in Indian elderly patients with STEMI.
Definite Improvement and A Welcome Addition to the Physician's Armamentarium with Tenecteplase in STEMI26
Aim
A post-licensure, observational, prescription-event monitoring study was conducted to assess the safety and effectiveness of single intravenous bolus administration of tenecteplase in the management of patients presenting with STEMI in clinical practice.
Design and Methods
Data of 6000 patients who had ST-elevation myocardial infarction and received weight-adjusted tenecteplase injection was analyzed.
Results
- The success rate was higher when treated early (within 3 hrs) and majority of patients (93.93%) achieved clinically successful thrombolysis (see Table 6).
- The overall mortality was 3.23%. The elderly (≤65 years; 24.58%) and diabetics (38.2%) had clinically successful thrombolysis of 87.73% and 90.49% respectively.
- The overall incidences of intracranial hemorrhage (ICH), severe bleeding, stroke and ventricular tachyarrhythmia were 0.62%, 3.18%, 0.12% and 3.07% respectively and were not significantly different in females, diabetics and elderly patients. This data after analysis shows that the tenecteplase causes clinically successful thrombolysis in 85-89% people treated within 6 hours and delay in the same is associated with increased incidence of heart failure, ventricular tachyarrhythmia and mortality.
Conclusion
Tenecteplase shows high efficacy and safety in-hospital use and this is a definite improvement in and a welcome addition to the Physician's armamentarium in the management of STEMI.
Efficacy and Safety of Tenecteplase in Patients with STEMI27
Tenecteplase is safe and effective in Indian patients with STEMI and conforms to the international ASSENT-2 trial data. This was confirmed in a study conducted to assess the efficacy and safety of tenecteplase in Indian patients with STEMI. Cardiologists/physicians who had used tenecteplase for management of STEMI, recorded safety and efficacy parameters from consecutively treated patients.
As per the prescribing information, tenecteplase was administered. As recommended by guidelines and as routine clinical practice, adjunctive therapy with clopidogrel and UFH/LMWH was administered.
A total of 507 patients with mean age of 58.28 ±12.23 yrs with STEMI were enrolled in the study and were treated with weight-adjusted tenecteplase within median chest pain to drug interval of 120 minutes.
Results from the study reported that in 436 patients with median duration required for ≥50% resolution of ST segment of 75 minutes, resolution of chest pain within median interval of 45 minutes. Clinically successful thrombolysis was reported in 80.67% patients. Incidence of intra-cranial hemorrhage attributable to tenecteplase was 0.39% and myocardial re-infarction was 2.96%.
Prescribing Information
Description
Tenecteplase is an enzyme (serine protease) that converts plasminogen to plasmin, primarily in the presence of fibrin. Tenecteplase binds to fibrin in a thrombus and selectively converts thrombus bound plasminogen to plasmin. This initiates local proteolysis of the fibrin matrix of the thrombus. It is a modified form of the naturally occurring human plasminogen activator. The molecule exhibits a well-known pronounced domain structure. Three sites of the molecule have been modified using site directed mutagenesis, thus tenecteplase has different pharmacokinetic properties from those of r-tPA.
Dosage Form and Strength
Tenecteplase is available as vials containing lyophilized product in the strengths of 30 mg (6000 IU), 40mg(8000 IU) and 50mg(10,000 IU) respectively.
Therapeutic Indication
Tenecteplase is indicated in adults for thrombolytic treatment of suspected myocardial infarction with persistent ST segment elevation or recent left Bundle Branch Block within 6 hours after the onset of acute myocardial infarction (AMI) symptoms. Treatment should be initiated as soon as possible after the onset of AMI symptoms.
Posology and Method of Administration
Posology
Tenecteplase should be prescribed by physicians experienced in the use of thrombolytic treatment and with the facilities to monitor that use. Treatment should be initiated as soon as possible after the onset of AMI symptoms.
Tenecteplase is for intravenous administration only. The recommended total dose should not exceed 50 mg and is based upon patient weight. A single bolus dose should be administered over 5 to 10 seconds based on patient weight. Treatment with tenecteplase should be initiated as soon as possible after TM onset of symptoms. The weight-based dosage is shown in Table 7.
|
Patient’s weight (kg)
|
Dose (mg) |
|
< 60 |
30 mg |
|
≥ 60 to < 70 |
35 mg |
|
≥ 70 to < 80 |
40 mg |
|
≥ 80 to < 90 |
45 mg |
|
≥ 90 |
50 mg |
Reconstitution and Method of Administration
- Tenecteplase should be reconstituted by adding the 6/8/10 ml of water for injections to the vial containing 30/40/50mgpowder respectively, to make the final concentration of 5 mg/ml for any strength.
- Ensure that the appropriate vial strength is chosen according to the body weight of the patient.
- Check that the cap of the vial is still intact.
- Remove the flip-off cap from the vial.
- Add the water for injections into the vial slowly to avoid foaming.
- Reconstitute by swirling gently.
- The reconstituted preparation is a colourless to pale yellow, clear solution. Only clear solution without particles should be used.
- Transfer the appropriate volume of reconstituted solution of tenecteplase into the syringe, based on the patient's weight.
- Tenecteplase should be administered to the patient, intravenously over 5 to 10 seconds.
- A pre-existing intravenous line may be used for administration of tenecteplase in 0.9% sodium chloride solution only. Tenecteplase is incompatible with dextrose solution. No other medicinal product should be added to the injection solution.
- It should not be administered into a line containing dextrose. Precipitation may occur when tenecteplase is administered in an IV line containing dextrose. Dextrose-containing lines should be flushed with a saline-containing solution prior to and following single bolus administration of tenecteplase.
- Any unused solution should be discarded.
Adjunctive Therapy
Antithrombotic adjunctive therapy with platelet inhibitors and anticoagulants should be administered according to the current relevant treatment guidelines for the management of patients with ST-elevation myocardial infarction.
Contraindications
Tenecteplase must not be administered to patients with a history of an anaphylactic (i.e. life-threatening) reaction to any of the constituents (i.e. tenecteplase or any excipient). If treatment with tenecteplase is nevertheless considered to be necessary, facilities for resuscitation should be immediately available in case of need.
Furthermore, tenecteplase is contraindicated in the following situations because thrombolytic therapy is associated with a higher risk of bleeding:
- Significant bleeding disorder or diathesis either at present or within the past 6 months
- Patients receiving effective oral anticoagulant treatment, e.g. warfarin sodium (INR>1.3)
- Any history of central nervous system damage (i.e. neoplasm, aneurysm, intracranial or spinal surgery)
- Known haemorrhagic diathesis
- Severe uncontrolled hypertension
- Intracranial or intraspinal surgery or trauma within 2 months or major surgery, biopsy of a parenchymal organ, or significant trauma within the past 2 months (this includes any trauma associated with the current AMI)
- Recent trauma to the head or cranium or history of cerebrovascular accident
- Prolonged cardiopulmonary resuscitation (> 2 minutes) within the past 2 weeks
- Acute pericarditis and/or subacute bacterial endocarditis
- Acute pancreatitis
- Severe hepatic dysfunction, including hepatic failure, cirrhosis, portal hypertension (oesophageal varices) and active hepatitis
- Active peptic ulceration
- Arterial aneurysm and known arterial/venous malformation
- Neoplasm with increased bleeding risk
- Any known history of haemorrhagic stroke or stroke of unknown origin
- Known history of ischaemic stroke or transient ischaemic attack in the preceding 6 months
- Dementia
Special Warnings and Precautions for Use
Warnings
Bleeding
The most common complication encountered during tenecteplase therapy is bleeding. The type of bleeding associated with thrombolytic therapy can be divided into two types:
- Internal bleeding, involving intracranial and retroperitoneal sites, or the gastrointestinal, genitourinary, or respiratory tracts.
- Superficial or surface bleeding, observed mainly at vascular puncture and access sites (e.g., venous cutdowns, arterial punctures) or sites of recent surgical intervention.
The concomitant use of heparin anticoagulation may contribute to bleeding. As fibrin is lysed during tenecteplase therapy, bleeding from recent puncture site may occur. Therefore, thrombolytic therapy requires careful attention to all possible bleeding sites (including catheter insertion sites, arterial and venous puncture sites, cutdown sites and needle puncture sites).
Intramuscular injections and non-essential handling of the patient should be avoided for the first few hours following treatment with tenecteplase. Venipunctures should be performed and monitored carefully. Should an arterial puncture be necessary during the first few hours following tenecteplase therapy, it is preferable to use an upper extremity vessel that is accessible to manual compression. Pressure should be applied for at least 30 minutes, a pressure dressing applied, and the puncture site checked frequently for evidence of bleeding. Most frequently haemorrhage at the injection site, and occasionally genitourinary and gingival bleeding were observed. Should serious bleeding occur, in particular cerebral haemorrhage, concomitant heparin administration should be terminated immediately. Administration of protamine should be considered if heparin has been administered within 4 hours before the onset of bleeding. In the few patients who fail to respond to these conservative measures, judicious use of transfusion products may be indicated. Transfusion of cryoprecipitate, fresh frozen plasma, and platelets should be considered with clinical and laboratory reassessment after each administration. A target fibrinogen level of 1 g/l is desirable with cryoprecipitate infusion. Antifibrinolytic agents are available as a last alternative.
Each patient being considered for therapy with tenecteplase should be carefully evaluated and anticipated benefits weighed against potential risks associated with therapy. In the following conditions, the risk of tenecteplase therapy may be increased and should be weighed against the anticipated benefits:
- Systolic blood pressure >160 mmHg and/or diastolic BP >110 mmHg
- Cerebrovascular disease
- Recent gastrointestinal or genitourinary bleeding (within the past 10 days)
- High likelihood of left heart thrombus, e.g., mitral stenosis with atrial fibrillation
- Any known recent (within the past 2 days) intramuscular injection
- Advanced age, i.e. over 75 years
- Low body weight <60 kg
- Recent major surgery, e.g., coronary artery bypass graft, obstetrical delivery, organ biopsy, previous puncture of non-compressible vessels
- Recent trauma
- Acute pericarditis
- Subacute bacterial endocarditis
- Hemostatic defects, including those secondary to severe hepatic or renal disease
- Severe hepatic dysfunction
- Pregnancy
- Diabetic hemorrhagic retinopathy or other hemorrhagic ophthalmic conditions
- Septic thrombophlebitis or occluded arterio-venous cannula at seriously infected site
- Patients currently receiving oral anticoagulants, e.g., warfarin sodium
- Recent administration of GP IIb/IIIa inhibitors
- Any other condition in which bleeding constitutes a significant hazard or would be particularly difficult to manage because of its location
- Patients receiving oral anticoagulants: The use of tenecteplase may be considered when dosing or time since the last intake of anticoagulant treatment makes residual efficacy unlikely and if appropriate test(s) of anticoagulant activity for the product(s) concerned show no clinically relevant activity on the coagulation system (e.g. INR ≤ 1.3 for vitamin K antagonists or other relevant test(s) for other oral anticoagulants are within the respective upper limit of normal).
Arrhythmias
Coronary thrombolysis may result in arrhythmias associated with reperfusion. These arrhythmias (such as sinus bradycardia, accelerated idioventricular rhythm, ventricular premature depolarizations, ventricular tachycardia) are not different from those often seen in the ordinary course of acute myocardial infarction and may be managed with standard anti-arrhythmic measures. It is recommended that anti-arrhythmic therapy for bradycardia and/or ventricular irritability be available when tenecteplase is administered.
Cholesterol Embolization
Cholesterol embolism has been reported rarely in patients treated with all types of thrombolytic agents; the true incidence is unknown. This serious condition, which can be lethal, is also associated with invasive vascular procedures (e.g., cardiac catheterization, angiography, vascular surgery) and/or anticoagulant therapy. Clinical features of cholesterol embolism may include livedo reticularis, “purple toe” syndrome, acute renal failure, gangrenous digits, hypertension, pancreatitis, myocardial infarction, cerebral infarction, spinal cord infarction, retinal artery occlusion, bowel infarction and rhabdomyolysis.
Use with Percutaneous Coronary Intervention (PCI)
In patients with large ST segment elevation myocardial infarction, physicians should choose either thrombolysis or PCI as the primary treatment strategy for reperfusion. If primary PCI is scheduled according to the current relevant treatment guidelines, tenecteplase should not be given. Patients who cannot undergo primary PCI within one hour as recommended by guidelines and receive tenecteplase as primary coronary recanalization treatment should be transferred without delay to a coronary intervention capable facility for angiography and timely adjunctive coronary intervention within 6-24 hours or earlier if medically indicated.
Precautions
General
Standard management of myocardial infarction should be implemented concomitantly with tenecteplase treatment. Arterial and venous punctures should be minimized. Non-compressible arterial puncture must be avoided and internal jugular and subclavian venous punctures should be avoided to minimize bleeding from the non-compressible sites. In the event of serious bleeding, heparin and antiplatelet agents should be discontinued immediately. Heparin effects can be reversed by protamine.
Re-administration
Re-administration of plasminogen activators, including tenecteplase to patients who have received prior plasminogen activator therapy has not been systematically studied. Although sustained antibody formation in patients receiving one dose of tenecteplase has not been documented, re-administration should be undertaken with caution. Caution is needed when administering tenecteplase to persons with a known hypersensitivity (other than anaphylactic reaction) to the active substance or to any of the excipients. If an anaphylactoid reaction occurs, the injection should be discontinued immediately and appropriate therapy should be initiated. In any case, tenecteplase should not be re-administered before assessment of haemostatic factors like fibrinogen, plasminogen and alpha2-antiplasmin.
Drug/Laboratory Test Interactions
During tenecteplase therapy, results of coagulation tests and/or measures of fibrinolytic activity may be unreliable unless specific precautions are taken. Tenecteplase is an enzyme that, when present in blood in pharmacologic concentrations, remains active under in vitro conditions. This can lead to degradation of fibrinogen in blood samples removed for analysis.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies in animals have not been performed to evaluate the carcinogenic potential, mutagenicity or the effect on fertility.
Drugs Interactions
Formal interaction studies of tenecteplase with other drugs have not been performed. Patients studied in clinical trials of tenecteplase were routinely treated with heparin and aspirin. Anticoagulants (such as heparin and vitamin K antagonists) and drugs that alter platelet function (such as acetylsalicylic acid, dipyridamole and GP IIb/IIIa inhibitors) may increase the risk of bleeding if administered prior to, during or after tenecteplase therapy.
Use in Special Populations
Pregnancy (Category C)
Tenecteplase has been shown to elicit maternal and embryo toxicity in rabbits given multiple IV administrations. Subsequent embryonic deaths were secondary to maternal hemorrhage and no fetal anomalies were observed. Tenecteplase does not elicit maternal and embryo toxicity in rabbits following a single IV administration. Tenecteplase is not considered to be teratogenic. There are no adequate and well-controlled studies in pregnant women. Tenecteplase should be given to pregnant women only if the potential benefits justify the potential risk to the fetus.
Nursing Mothers
It is not known if tenecteplase is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when tenecteplase is administered to a nursing woman. Breast-feeding should be discarded within the first 24 hours after thrombolytic therapy.
Fertility
Clinical data as well as nonclinical studies on fertility are not available for tenecteplase.
Elder people (≥ 75 years)
Tenecteplase should be administered with caution in older people (≥75 years) due to a higher bleeding risk.
Paediatric population
The safety and efficacy of tenecteplase in children (below 18 years) have not been established. No data are available.
Effects on Ability to Drive and Use Machines
Not relevant. No information available
Undesirable Effects
Haemorrhage is a very common undesirable effect associated with the use of tenecteplase. The type of haemorrhage is predominantly superficial at the injection site. Ecchymoses are observed commonly but usually do not require any specific action. Death and permanent disability are reported in patients who have experienced stroke (including intracranial bleeding) and other serious bleeding episodes. Adverse reactions listed below are classified according to frequency and system organ class. Frequency groupings are defined according to the following convention: Very common (≥1/10), Common (≥1/100 to <1/10), Uncommon (≥1/1,000 to <1/100), Rare (≥1/10,000 to <1/1,000), Very rare (<1/10,000), Not known (cannot be estimated from the available data). Table 8 displays frequency of adverse reactions.
|
System Organ Class |
Adverse Reaction |
|
Immune system disorders |
|
|
Rare |
Anaphylactoid reaction (including rash, urticaria, bronchospasm, laryngeal oedema) |
|
Nervous system disorders |
|
|
Uncommon |
Intracranial haemorrhage (such as cerebral haemorrhage, cerebral haematoma, haemorrhagic stroke, haemorrhagic transformation stroke, intracranial haematoma, subarachnoid haemorrhage) including associated symptoms as somnolence, aphasia, hemiparesis, convulsion |
|
Eye disorders |
|
|
Uncommon |
Eye haemorrhage |
|
Cardiac disorders |
|
|
Uncommon |
Reperfusion arrhythmias (such as asystole, accelerated idioventricular arrhythmia, arrhythmia, extrasystoles, atrial fibrillation, atrioventricular first degree to atrioventricular block complete, bradycardia, tachycardia, ventricular arrhythmia, ventricular fibrillation, ventricular tachycardia) occur in close temporal relationship to treatment with tenecteplase. Reperfusion arrhythmias may lead to cardiac arrest, can be life threatening and may require the use of conventional antiarrhythmic therapies. |
|
Rare |
Pericardial haemorrhage |
|
Vascular disorders |
|
|
Very common |
Haemorrhage |
|
Rare |
Embolism (thrombotic embolisation) |
|
Respiratory, thoracic and mediastinal disorders |
|
|
Common |
Epistaxis |
|
Rare |
Pulmonary haemorrhage |
|
Gastrointestinal disorders |
|
|
Common |
Gastrointestinal haemorrhage (such as gastric haemorrhage, gastric ulcer haemorrhage, rectal haemorrhage, haematemesis, melaena, mouth haemorrhage) |
|
Uncommon |
Retroperitoneal haemorrhage (such as retroperitoneal haematoma) |
|
Not known |
Nausea, vomiting |
|
Skin and subcutaneous tissue disorders |
|
|
Common |
Ecchymosis |
|
Renal and urinary disorders |
|
|
Common |
Urogenital haemorrhage (such as haematuria, haemorrhage urinary tract) |
|
General disorders and administration site conditions |
|
|
Common |
Injection site haemorrhage, puncture site haemorrhage |
|
Investigations |
|
|
Rare |
Blood pressure decreased |
|
Not known |
Body temperature increased |
|
Injury, poisoning and procedural complications |
|
|
Not known |
Fat embolism, which may lead to corresponding consequences in the organs concerned |
As with other thrombolytic agents, the following events have been reported as sequelae of myocardial infarction and/or thrombolytic administration:
- very common (>1/10): hypotension, heart rate and rhythm disorders, angina pectoris
- common (>1/100, <1/10): recurrent ischaemia, cardiac failure, myocardial infarction, cardiogenic shock, pericarditis, pulmonary oedema
- uncommon (>1/1,000, <1/100): cardiac arrest, mitral valve incompetence, pericardial effusion, venous thrombosis, cardiac tamponade, myocardial rupture
- rare (>1/10,000, <1/1,000): pulmonary embolism
These cardiovascular events can be life-threatening and may lead to death.
Overdose
In the event of overdose there may be an increased risk of bleeding. In case of severe prolonged bleeding substitution therapy may be considered (plasma, platelets).
Pharmacological Properties
Mechanism of Action
Tenecteplase is a modified form of human tissue plasminogen activator (tPA) that binds to fibrin and converts plasminogen to plasmin. In the presence of fibrin, Tenecteplase conversion of plasminogen to plasmin is increased relative to its conversion in the absence of fibrin. This fibrin specificity decreases systemic activation of plasminogen and the resulting degradation of circulating fibrinogen as compared to a molecule lacking this property.
Pharmacodynamic Properties
Following administration of 30, 40, or 50 mg of tenecteplase, there are decreases in circulating fibrinogen and plasminogen. Tenecteplase has a higher fibrin specificity and greater resistance to inactivation by its endogenous inhibitor (Plasminogen activator inhibitor - PAI-1) compared to native t-PA. After administration of tenecteplase dose dependent consumption of alpha2-antiplasmin (the fluid-phase inhibitor of plasmin) with consequent increase in the level of systemic plasmin generation have been observed. This observation is consistent with the intended effect of plasminogen activation.
Pharmacokinetic Properties
In patients with acute myocardial infarction (AMI), tenecteplase administered as a single bolus exhibits a biphasic disposition from the plasma. Tenecteplase has an initial half-life of 20 to 24 minutes. Liver metabolism is the major clearance mechanism for Tenecteplase. In the phase III multicentric comparative study of manufacturer's tenecteplase, pharmacokinetic blood sampling was performed in the first 24 subjects (12 in each arm) enrolled in the study. One subject from manufacturer's tenecteplase arm and one subject from the innovator product arm (reference) were excluded from pharmacokinetic analysis. Statistical analysis was performed on the pharmacokinetic parameters by using SAS®, statistical software (Version: 9.3; SAS® Institute Inc., USA). Ratio analysis was performed for ln-transformed pharmacokinetic parameters Cmax, AUC0-8 and AUC0-∞. For manufacturer's tenecteplase and reference product, mean Cmax was 12440 and 10916 ng/mL, AUC0-∞ was 10815 and 8771 (ng X hr/mL) and AUC0-∞ was 10953 and 9023 (ng X hr/mL) respectively. The median Tmax observed for both manufacturer's tenecteplase and reference formulations was 5 minutes. The median t1/2 observed for manufacturer's tenecteplase and reference formulation was 0.84 hrs and 1.123 hrs respectively. The inter-subject variability of manufacturer's tenecteplase was much lower for Cmax compared to reference product. The inter-subject variability was comparable for AUC0-8 and AUC0-∞ between the two arms. The ratios of the mean of ln-transformed data (T/R ratio) for lnCmax, lnAUC0-8 and lnAUC0-∞ were 124.88, 124.50 and 121.94 respectively.
Incompatibilities
A pre-existing intravenous line may be used for administration of tenecteplase in 0.9% sodium chloride solution only. Tenecteplase is incompatible with dextrose solution. No other medicinal product should be added to the injection solution. Dextrose-containing lines should be flushed with a saline containing solution prior to and following single bolus administration of tenecteplase. Heparin should be administered in a separate intravenous line.
Packaging Information
Tenecteplase is available as a kit with vial containing lyophilized product in the strengths of 30 mg (6000 IU), 40mg(8000 IU) and 50mg(10,000 IU) respectively. Each kit also contains one SWFI (10 ml) unit, one disposable syringe (10 ml) with needle, one 21G needle and three alcohol swabs.
Storage and Handing Instructions
Tenecteplase vials (30 mg, 40 mg or 50 mg) are stable at 2-8 °C. Do not use beyond expiration date stamped on carton. Tenecteplase vials should be protected from direct sunlight. Do not freeze or shake.
Discard the vials, syringe and needles in appropriate containers.
Patient Counselling Information
Brand Name: Telyse TM
Generic Name: Tenecteplase (Pronunciation: ten EK te plase)
What is the most important information I should know about tenecteplase?
- What should I discuss with my health care provider before I receive tenecteplase?
- How is tenecteplase given?
- What happens if I miss a dose?
- What happens if I overdose?
- What should I know after receiving tenecteplase?
- What other drugs will affect tenecteplase?
- Where can I get more information?
What is tenecteplase?
- Tenecteplase is in a group of drugs called tissue plasminogen activators (TPAs). It works by causing the body to over-produce a substance called plasmin to dissolve unwanted blood clots.
- Tenecteplase is used to prevent death from a heart attack (acute myocardial infarction).
- Tenecteplase may also be used for other purposes not listed in this medication guide.
What are the possible side effects of tenecteplase?
- Get emergency medical help if you have any of these signs of an allergic reaction: hives; difficulty breathing; swelling of your face, lips, tongue, or throat.
- Tell your caregivers at once if you have a serious side effect such as:
- blood in your urine or stools; nosebleed, coughing up blood;
- bleeding from a recent injury or surgery incision;
- bleeding around the IV needle;
- fast, slow, or uneven heart rate; or
- feeling like you might pass out.
Rare but serious side effects may include:
- purple discoloration of your legs or toes;
- severe pain in your upper stomach spreading to your back, nausea and vomiting, fast heart rate;
- sudden numbness, weakness, headache, confusion, or problems with vision, speech, or balance;
- chest pain or heavy feeling, pain spreading to the arm or shoulder, nausea, sweating, general ill feeling;
- swelling, weight gain, feeling short of breath, urinating less than usual or not at all;
- drowsiness, confusion, mood changes, increased thirst, loss of appetite, nausea and vomiting;
- muscle pain or tenderness with fever or flu symptoms and dark colored urine;
- pain or unusual sensations in your back;
- numbness, weakness, or tingly feeling in your legs or feet;
- muscle weakness or loss of use; or
- loss of bowel or bladder control.
Less serious side effects may include mild nausea or dizziness.
This is not a complete list of side effects and others may occur. Tell your doctor about any unusual or bothersome side effect.
What is the most important information I should know about tenecteplase?
- You should not receive this medication if you have internal bleeding, brain cancer or aneurysm, a history of stroke, a bleeding or blood clotting disorder, or if you have had brain or spinal cord injury or surgery within the past 2months.
- Before you are treated with tenecteplase, tell your doctor if you have a blood vessel disorder of the eye, severe liver or kidney disease, high blood pressure, an infection of the lining of your heart (also called bacterial endocarditis), a recent history of stomach or urinary bleeding, if you have recently had a baby, or if you have recently had a serious injury or major surgery.
- Also tell your doctor if you take a blood thinner such as warfarin (Coumadin), or other medications to treat or prevent blood clots.
- Tell your caregivers at once if you have a serious side effect such as blood in your urine or stools, nosebleed, coughing up blood, bleeding from a skin wound or the IV needle, fast or slow heart rate, or feeling like you might pass out.
- Tenecteplase may cause rare but serious side effects such as: purple discoloration of your legs or toes, sudden numbness or weakness, problems with vision or speech, chest pain or heavy feeling, urinating less than usual or not at all, muscle pain or tenderness, dark colored urine, unusual sensations in your back, numbness or tingling in your legs or feet, muscle weakness or loss of use, or loss of bowel or bladder control.
- Tenecteplase can cause you to have unusual results with blood tests. Tell any doctor who treats you that you have recently received tenecteplase.
What should I discuss with my health care provider before I receive tenecteplase?
- You should not receive this medication if you have:
- internal bleeding;
- a history of stroke;
- brain cancer;
- brain aneurysm;
- a bleeding or blood clotting disorder (such as hemophilia); or
- if you have had brain or spinal cord injury or surgery within the past 2months.
- If you have certain conditions, you may need a dose adjustment or special tests to safely receive this
medication. Before you receive tenecteplase, tell your doctor if you have:
- a blood vessel disorder of the eye;
- severe liver or kidney disease;
- high blood pressure;
- an infection of the lining of your heart (also called bacterial endocarditis);
- a recent history of bleeding in your stomach, intestines, or urinary tract;
- if you have recently had a baby; or
- if you have recently had a serious injury or major surgery.
FDA pregnancy category C. Tenecteplase may be harmful to an unborn baby. Before you receive this medication, tell your doctor if you are pregnant.
It is not known whether tenecteplase passes into breast milk or if it could harm a nursing baby. Before you receive this medication, tell your doctor if you are breast-feeding a baby.
How is tenecteplase given?
- Tenecteplase is given as an injection through a needle placed into a vein. You will receive this injection in a hospital or emergency setting. It is usually given as soon as possible after the first signs of heart attack occur.
- Tenecteplase can cause you to have unusual results with blood tests. Tell any doctor who treats you that you have recently received tenecteplase.
What happens if I miss a dose?
- Tenecteplase is usually given as a single dose, so you are not likely to be on a dosing schedule.
What happens if I overdose?
- Since tenecteplase is given by a healthcare professional, you are not likely to receive an overdose. Symptoms of a tenecteplase overdose are not known.
What should I know after receiving tenecteplase?
- Follow your doctor's instructions about any restrictions on food, beverages, or activity after you receive tenecteplase.
What other drugs will affect tenecteplase?
- Tell your doctor about all other medications you have recently used or received, especially:
- a blood thinner such as warfarin;
- abciximab;
- alteplase;
- anistreplase;
- dipyridamole;
- eptifibatide;
- heparin;
- streptokinase;
- tirofiban; or
- urokinase.
This list is not complete and there may be other drugs that can interact with tenecteplase. Tell your doctor about all your prescription and over-the-counter medications, vitamins, minerals, herbal products, and drugs prescribed by other doctors. Do not start a new medication without telling your doctor.
Where can I get more information?
- Your doctor can provide more information about tenecteplase.
Remember, keep this and all other medicines out of the reach of children, never share your medicines with others, and use this medication only for the indication prescribed.
References
- Overbaugh KJ. Acute coronary syndrome. AJN. 2009;109(5):42-52.
- Guha S, Rishi Sethib, Rayc S, et al. Cardiological Society of India: Position statement for the management of ST elevation myocardial infarction in India. Indian Heart J. 2017;69:S63–S97.
- Singh A and Grossman SA. Acute coronary syndrome. Available at: https://www.ncbi.nlm.nih.gov/books/NBK459157/ Accessed on: 31st July 2019.
- Heat attack (myocardial infarction). Available at: https://www.health.harvard.edu/a_to_z/heart-attack-myocardial-infarction-a-to-z Accessed on: 31st July 2019.
- Heat attack. Available at: https://www.mayoclinic.org/diseases-conditions/heart-attack/symptoms-causes/syc-20373106 Accessed on: 31st July 2019.
- Sheridan PJ and Crossman DC. Critical review of unstable angina and non-ST elevation myocardial infarction. Postgrad Med J. 2002;78:717–726.
- Foth C and Mountfort S. Acute myocardial infarction ST elevation (STEMI). Available at: https://www.ncbi.nlm.nih.gov/books/NBK532281/ Accessed on: 31st July 2019.
- Myocardial infarction with STsegment-elevation: The acute management of myocardial infarction with ST-segment-elevation. January 2013. Available at: https://www.nice.org.uk/guidance/cg167/documents/myocardial-infarction-with-stsegmentelevation-stemi-full-version2
- Prabhakaran D, Jeemon P and Roy A. Cardiovascular diseases in India: Current epidemiology and future directions. Circulation. 2016;133:1605–1620.
- Singh SS, Paul SK, Pal R, et al. Acute coronary syndrome-related mortality audit in a teaching hospital at Port Blair, India. J Family Med Prim Care. 2017; 6(3): 502–508.
- Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2018;39:119–177.
- Barstow C and Rice M. Acute Coronary Syndrome: Diagnostic Evaluation. Am Fam Physician. 2017;95(3):170-177.
- Hanna EB and Glancy DL. ST-segment elevation: Differential diagnosis, caveats. Cleveland Clinic Journal Of Medicine. 2015;82(6):373-384.
- Mollon L and Bhattacharjee S. Health related quality of life among myocardial infarction survivors in the United States: A propensity score matched analysis. Health Qual Life Outcomes. 2017;15:235.
- Mishra S, Ramakrishnana S, Babu AS, et al. Management algorithms for acute ST elevation myocardial infarction in less industrialized world. Ind Heart J. 2017;69:S98–S103.
- Prescribing Information of TELYSE as on 31st July 2019.
- Cannon CP, Gibson CM, McCabe CH, et al. TNK-tissue plasminogen activator compared with front-loaded alteplase in acute myocardial infarction: Results of the TIMI 10B trial. Thrombolysis in Myocardial Infarction (TIMI) 10B Investigators. Circulation. 1998;98(25):2805-14.
- Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT-2) Investigators, Van De Werf F, Adgey J, Ardissino D, et al. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial.Lancet. 1999;354(9180):716-22.
- Armstrong PW, Gershlick A, Goldstein P, et al. The Strategic Reperfusion Early After Myocardial Infarction (STREAM) study. Am Heart J. 2010;160(1):30-35.e1.
- Armstrong PW, Gershlick AH, Goldstein P, et al. Fibrinolysis or primary PCI in st-segment elevation myocardial infarction. N Engl J Med. 2013;368:1379-1387.
- Cantor WJ, Fitchett D, Borgundvaag B, et al. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med. 2009;360:2705-18.
- Victor SM, Subban V, Alexander T, et al. A prospective, observational, multicenter study comparing tenecteplase facilitated PCI versus primary PCI in Indian Patients with STEMI (STEPP – AMI). J American Coll Cardiol. 2014;63(12 Supplement 2).
- Victor SM, Vijayakumar S, Alexander T, et al. Two-year follow-up data from the STEPP-AMI study: A prospective, observational, multicenter study comparing tenecteplase-facilitated PCI versus primary PCI in Indian patients with STEMI. Indian Heart J. 2016;68(2):169-73.
- Iyengar SS, Nair T, Hiremath J, et al. Pharmacological Reperfusion Therapy with Tenecteplase in 7,668 Indian Patients with ST Elevation Myocardial Infarction - A Real World Indian Experience. J Assoc Physicians India. 2017;65(9):43-47
- Sathyamurthy I, Bahuleyan CG, Srinivas A, et al. Efficacy and safety of tenecteplase in Indian elderly STEMI patients from the Elaxim Indian Registry.Indian Heart J. 2011;63(3):234-6.
- Iyengar SS, Nair T, Hiremath JS, et al. Efficacy & safety of tenecteplase in 6000 patients with ST-elevation myocardial infarction from the Elaxim Indian Registry.Indian Heart J. 2011;63(1):104-7.
- Sathyamurthy I, Srinivasan KN, Jayanthi K, et al. Efficacy and safety of tenecteplase in Indian patients with ST-segment elevation myocardial infarction.Indian Heart J. 2008;60(6):554-7.