A 47-year-old male was admitted from another intensive care unit (ICU) due to multiple chest injuries after a car accident, respiratory tract infection, and ICU polyneuropathy.
Xylistin_Monograph
Case Study
Presentation
Diagnosis
Based on clinical and microbiological evidence, severe pneumonia was confirmed.
Treatment
Antibiotics were initiated on the day 2 of the ICU stay. Combination of imipenem/cilastatin (10 days), gentamicin (22 days), ofloxacin (6 days) and vancomycin (14 days) were given.
Imipenem/cilastatin substituted with piperacillin/tazobactam for 12 days and piperacillin/ tazobactam with ceftriaxone for 10 days. On day 30, teicoplanin was added to the regimen given for 21 days.
On day 40 of the ICU stay, a Pandrug resistant (PDR) Pseudomonas aeruginosa strain was isolated from his bronchial secretions.
He was subsequently treated, due to the persistence of the infection, with a prolonged course of intravenous colistin for 52 days, combined with imipenem/cilastatin for 12 days, which was followed by meropenem for 23 days, and then ofloxacin for 16 days.
On day 54 a strain of Pseudomonas aeruginosa sensitive only to colistin (COS) was isolated from his bronchial secretions. The infection was gradually controlled and finally, the patient was discharged from the hospital in good condition.
Rediscovery of a Forgotten Antibiotic
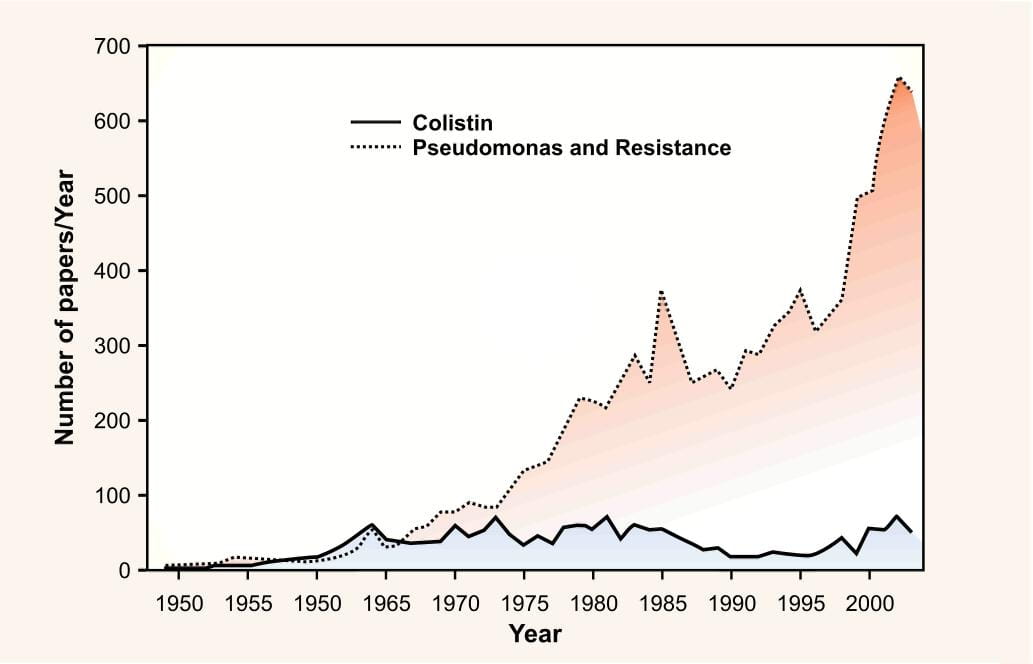
The antibiotic colistin was discovered almost 50 years ago, but was relegated due to apparent toxicity, in the presence of equally effective but safer alternatives like aminoglycosides and cephalosporins. However over the past few years, there has been a tremendous rise in the number of published articles addressing the use of colistin.
This trend is an indirect reflection of a new threat of resistance caused due to a worldwide spread of difficult to treat multi and pan drug resistant pathogens, the latter being defined as those resistant to all the available antibacterials, except polymyxins.
In particular, there is substantial concern worldwide with the mounting prevalence of infections caused by multidrug-resistant (MDR) Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae, which possess extended-spectrum beta-lactamases.
Carbapenems were often used as a last resort for the treatment of the aforementioned infections, because of their frequent resistance to fluoroquinolones, aminoglycosides and third generation cephalosporins. However, over the last decade there has been an alarming increase in reports of carbapenem resistance.
These trends are of particular concern given a dearth of new antibacterials on the horizon that specifically target gram-negative bacteria. The Food and Drug Administration (FDA) approval of new antimicrobial agents has decreased by 56% overthe past 20 years2 which eventually led to the "revival of colistin" use in human medicine.
The Increasing Challenge of Gram-negative Infections
- Gram-negative pathogens present a growing challenge in the treatment of hospital-acquired infections.
- Resistance to most antipseudomonal agents has increased by >20%, over the five years.
- Notably, resistance to carbapenems is significantly increasing and have witnessed the emergence of carbapenemases, metallo-betalactamases as well as pan-resistant Gram-negative bacilli; globally as well as in India.
- Hospital-acquired infections and antibiotic resistance negatively impact clinical outcomes and escalate healthcare costs.
- Today's patients have more risk factors for acquiring resistant pathogens.
- No new classes of agents for the treatment of Gram-negative infections are on the horizon.
Gram Negative Resistance: A Healthcare Crisis
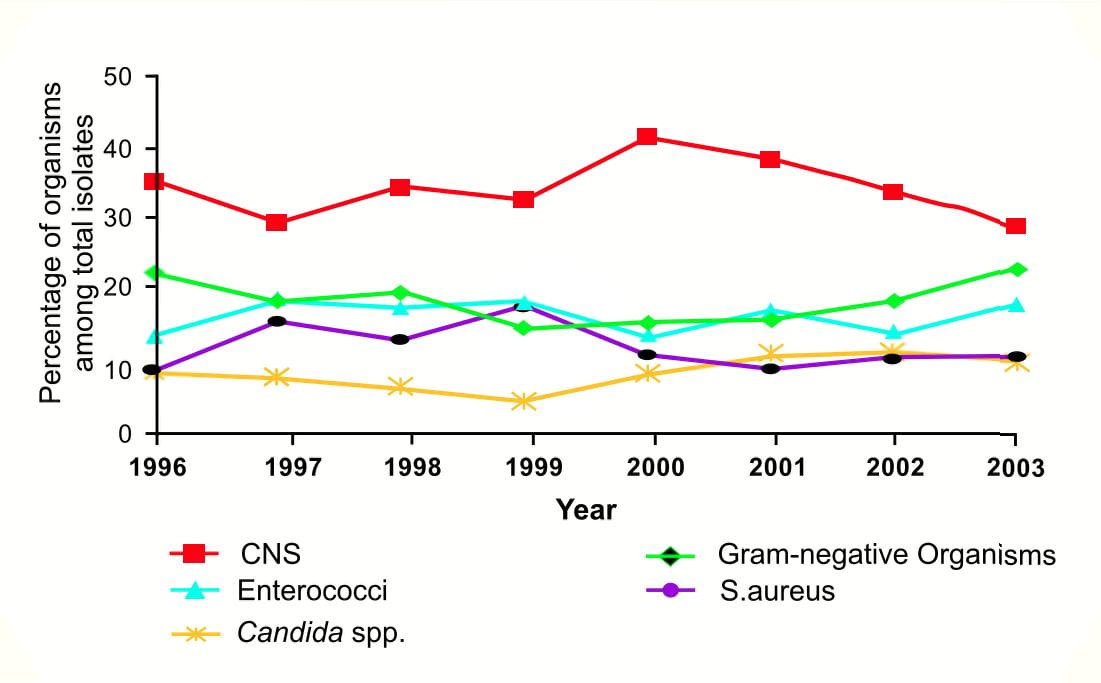
Though gram positive bacteria are assumed to be a major cause of nosocomial infections in the West, recent reports worldwide suggest the re-emergence of Gram-negative bacteria. In a 7-year epidemiologic study conducted at the University of Pennsylvania, the proportion of primary healthcare-associated bloodstream infections caused by gram-negative bacteria increased from 15.9% in 1999 to 24.1% in 20032.
Though no specific organism contributed to the overall rise in infections due to gram-negative bacteria, Pseudomonas aeruginosa, Acinetobacter baumannii, Klebsiella pneumoniae and E.coli accounted forover70% of infections.
Similarly, data analysed by Gaynes R et al3 from the National Nosocomial Infection Surveillance (NNIS) System [now the National Healthcare Safety Network (NHSN)] from 1986 to 2003,acknowledged gram-negative bacilli as a major cause of hospital-acquired infections in the ICUs.

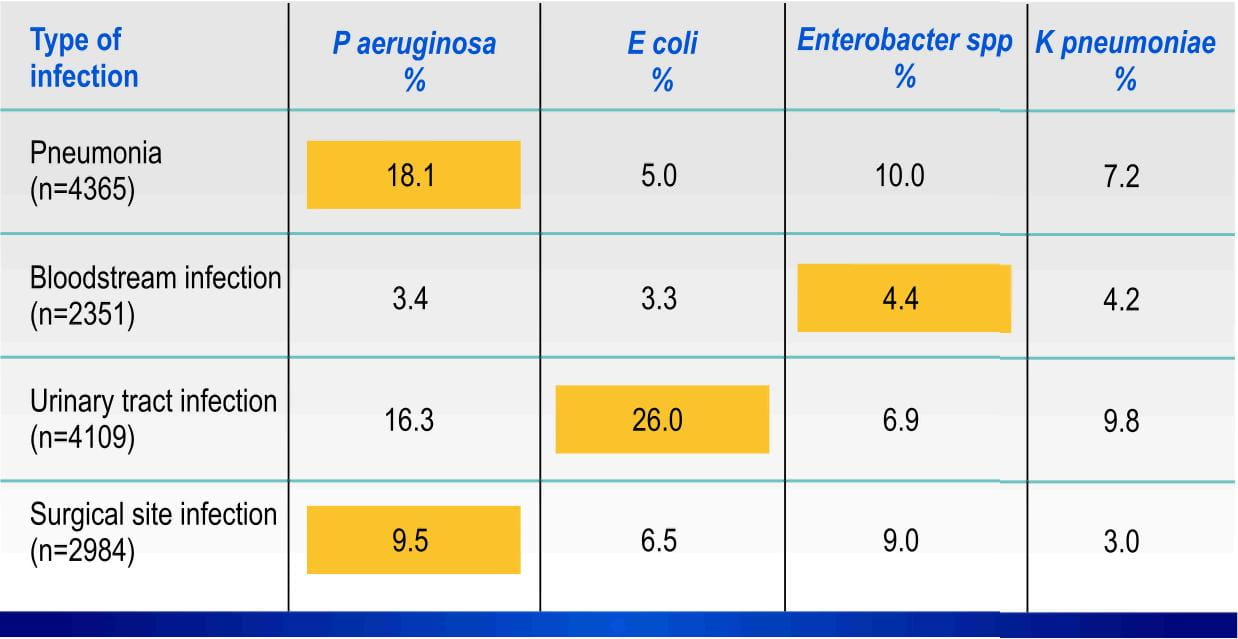
Pseudomonas aeruginosa accounted for the most common gram negative isolate from patients with ICU pneumonia.
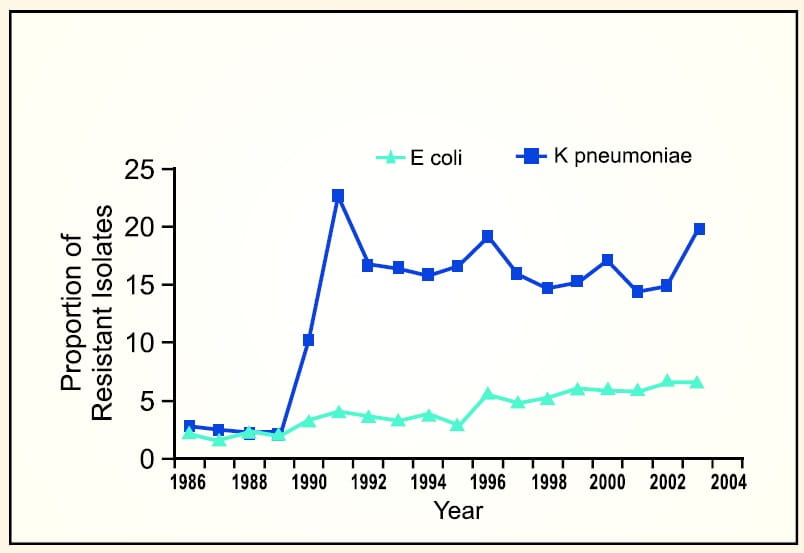
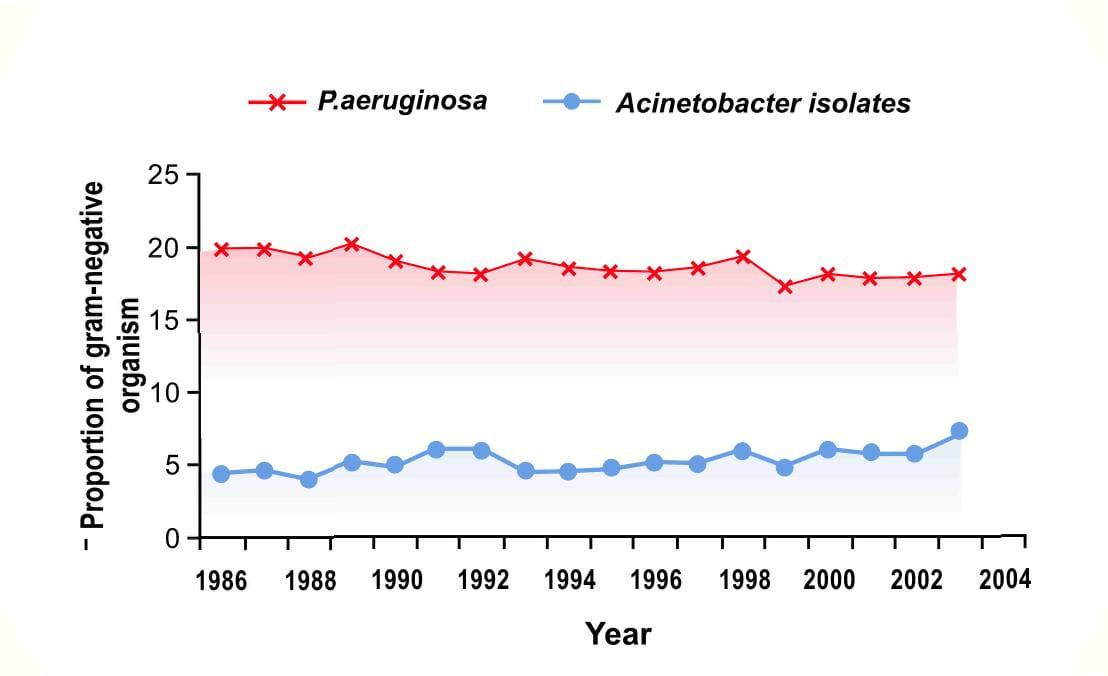
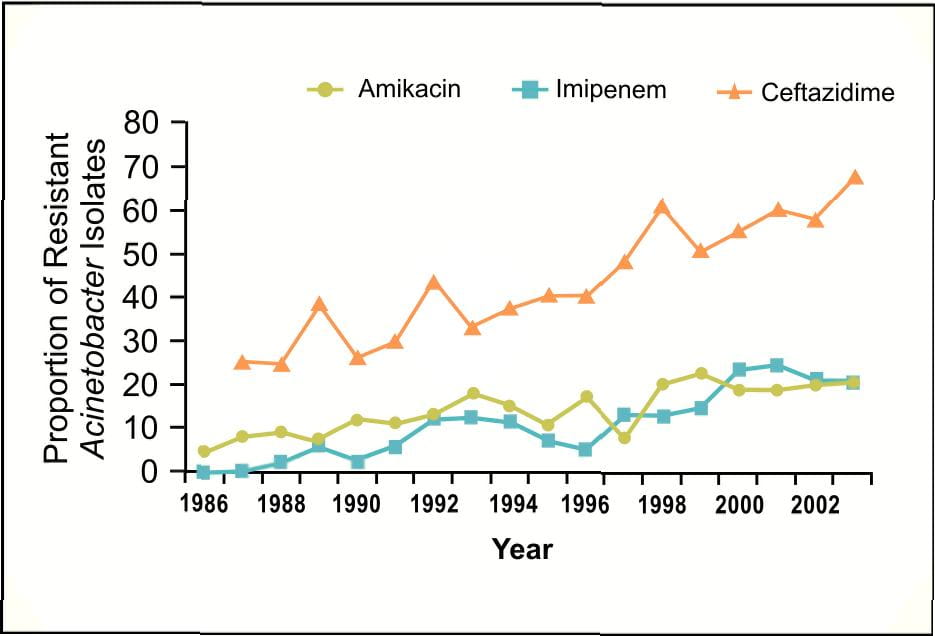
Acinetobacter baumannii which was once thought to be uncommon, has now emerged as an important pathogen worldwide in hospitalized patients causing high mortality. National data from the NNIS3 indicate that the incidence of Acinetobacter spps in pneumonia has increased by 75% from 1986 to 2003; p<0.001 (Figure 3).
Each of these Gram-negative bacteria has now been identified in the "Bad Bugs, No Drugs" initiative of the Infectious Disease Society of America (IDSA) as among the most worrisome, given the rising prevalence of antimicrobial resistance.
Although infections caused by them can strike anyone - the young and the old, the healthy and the chronically ill - they are especially grave for patients whose immune systems are compromised, such as those with AIDS, or in hospitalized patients who are critically ill. As long as there are options to switch from one class of antibiotics to another, the "resistance problem" can still be overcome. In the past, new drugs were able to help us out after we "lost" older antibiotics, e.g. amoxicillin, to resistance.
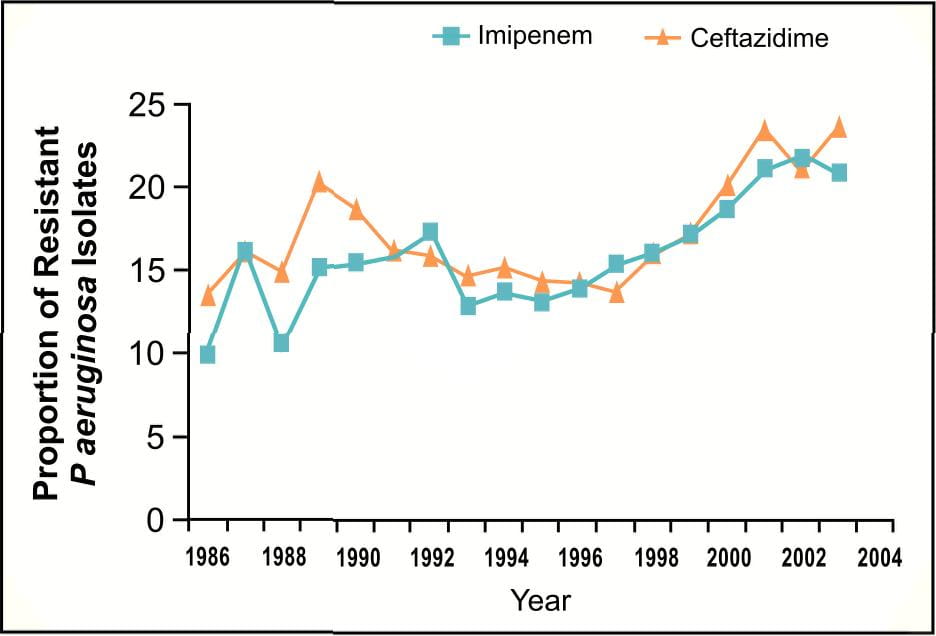
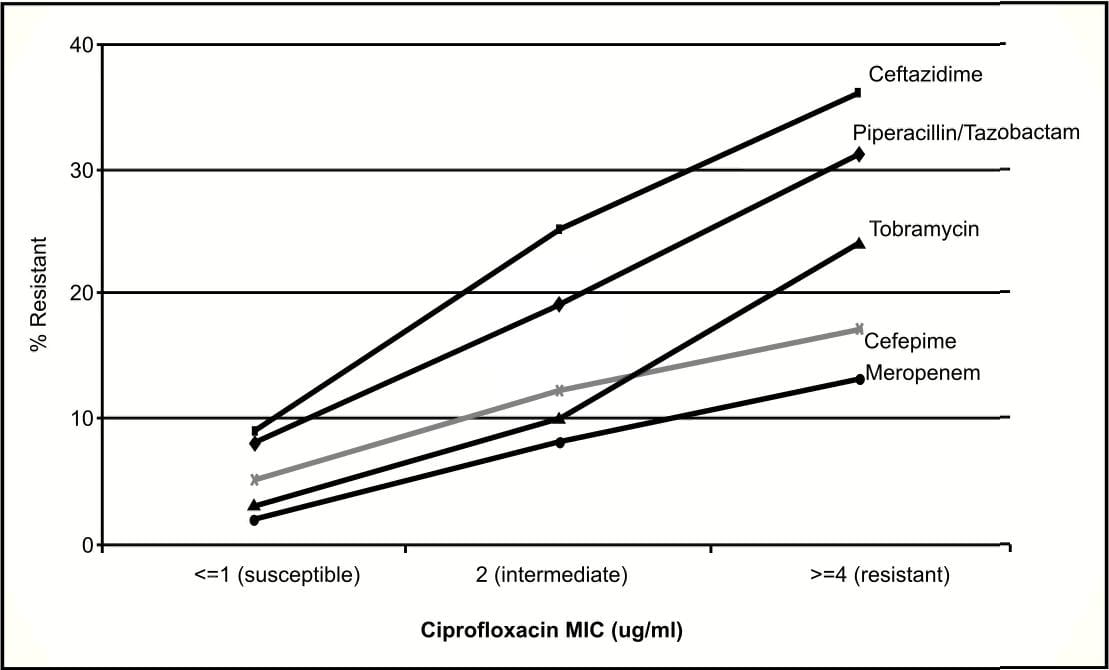
However, in 2003 tremendous increase in the prevalences of carbapenem-resistant Pseudomonas aeruginosa (Figure 4), third-generation cephalosporinresistant Pseudomonas aeruginosa and third-generation cephalosporin-resistant Klebsiella pneumoniae3 among clinical isolates from patients in ICU have been reported.
Global Scenario
Furthermore, significant increases in the resistance of Acinetobacter to amikacin and ceftazidime have been noted in 2003, with resistance to imipenem in prevalence increased from 0% to 42% in 20034.
A recent report described a "resistant island" containing 45 resistance genes within the acinetobacter genome5.
Of further concern is the rising incidence of Pseudomonas exhibiting multi-antibiotic resistance.
These data indicate that resistance to one agent is likely to have a higher prevalence of co-resistance across several antimicrobial classes6.
A recent review by Archibald7 showed that the types of pathogens encountered in healthcare institutions in North America, Europe, Asia, Australia and even Africa are, for the most part, similar. This suggests that the reservoirs of these pathogens, which are often the patients themselves, and the risk factors for hospital-acquired infection, such as invasive devices, are also similarworldwide.
Indian Scenario
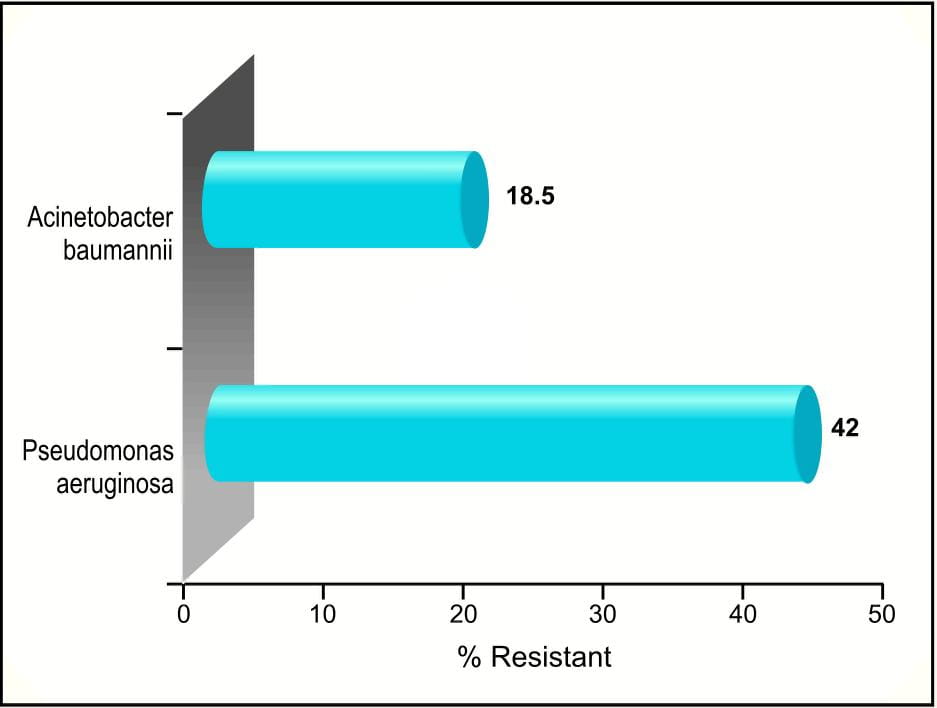
Analogous increase in carbapenem-resistant Pseudomonas aeruginosa, Acinetobacterbaumannii (Figure 8) and Enterobacteriaceae is being reported in our country, within a short span of carbapenem introduction (Meropenem 2002).
AIIMS: New Delhi8
PGIMER :Chandigarh9
The resistance to carbapenems especially in Pseudomonas spps results from reduced levels of drug accumulation or increased expression of efflux pump. However the novel menace of resistance has been ascribed to the production of metallo-beta lactamases (MBL)8.
MBL producers have been reported across India from Bangalore10 , Mahatma Gandhi Institute of Medical Sciences Maharashtra11, Chennai12, Chandigarh13, SARI Study group14 and CMC Vellore15 (Figure 10).
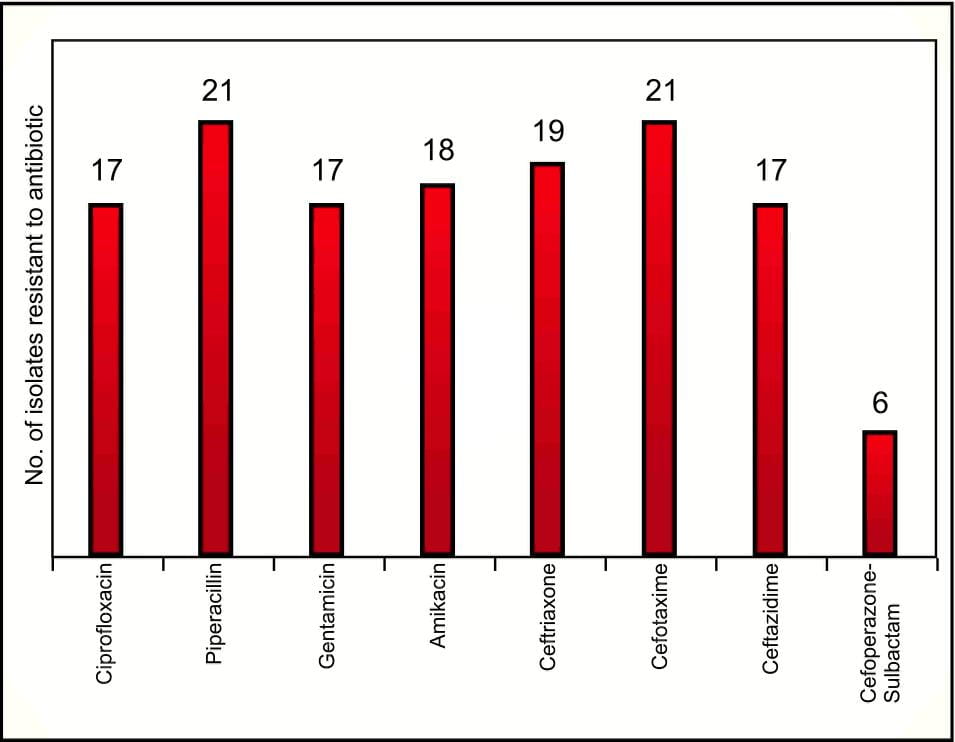
Most of these MBLs are susceptible to only aztreonam11 and colistin16 . Of further concern is the increasing prevalence of Gram-negative organisms resistant to all classes of antibacterials, including carbapenems. A study17 that included 150 clinical isolates of Acinetobacter spps, reported 21 isolates as resistant to meropenem. Of these 21 isolates, 28.57% were resistant to all antibiotics tested (Figure 11).
St. John's Medical College and Hospital, Bangalore.
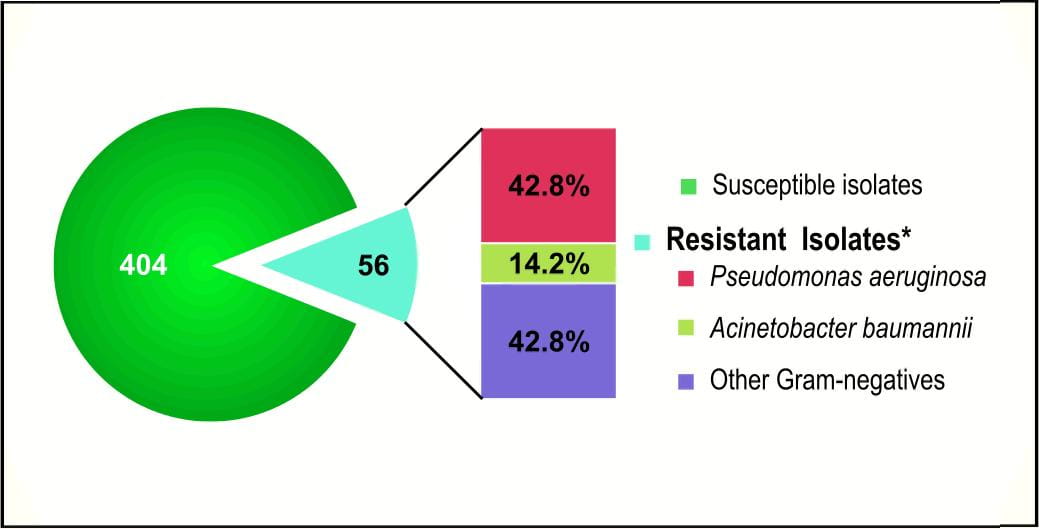
Similarly, a study conducted at CMC Vellore18, identified 12.2% of Pseudomonas aeruginosa, Acinetobacter baumannii and Enterobacteriacae as resistant to nine antibacterials tested (Figure 12).
CMC Vellore
This rapid emergence and dissemination of difficult-to-treat infections in hospitals worldwide is a problem of crisis dimensions.
For these species, polymyxins are sometimes the only available active antibiotics, which although highly active against gram-negative bacteria, were withdrawn in the 1970s because of reports of toxicity. Recent experiences suggest that polymyxins can be used to treat serious infections due to MDR Gram-negative bacteria without causing significant associated adverse events, based on the use of lower doses, avoidance of concurrent use of nephrotoxins, and improved fluid supplementation and supportive treatment.
We are fortunate to have this "Reserved" old class of polymyxin antibiotics (Polymyxin E) available for use as salvage therapy.
Xylistin (Colistimethate Sodium): Pharmacology at a Glance
- Polymyxin E is available as colistimethate sodium and colistin sulphate, the former being used as a parenteral as well aerosolized form.
- Colistimethate sodium is a prodrug, which is converted in vivo to colistin.
- Highly effective against MDR including carbapenem resistant Pseudomonas aeruginosa, Acinetobacter and baumannii and ESBLs.
- Exhibits rapid bactericidal and detergent-like action.
- Unique mode of action i.e. self promoted uptake results in dual benefits as in reduced resistance and enhanced synergistic effect with other antibacterials.
- Neutralizes endotoxin activity hence beneficial in sepsis.
- No cross resistance with existing armamentarium of antibacterials.
- Stable to all beta-lactamases including Amp-C and MBLs.
- Exhibits maximum synergy with piperacillin and ceftazidime against MDR Pseudomonas aeruginosa.
- Exhibits maximum synergy with meropenem, rifampicin and sulbactam against MDR Acinetobacter baumannii.
- Achieves high peak serum concentration of 18 mg/L after 10 minutes of intravenous administration.
- Eliminated predominately by kidneys.
Polymyxins: The Winning Antibiotics
The polymyxins are a group of polypepetide antibiotics, which were first isolated in 1947 from a spore-bearing soil bacillus (Bacillus polymyxa). Among numerous polymyxins (A, B, C, D and E), polymyxin B and polymyxin E have been used therapeutically. Polymyxin B is available as a parenteral formulation and is also found in combination topical antibiotics as the sulphate salt.
Polymyxin E (colistin) has two salt forms:
- Sulphomethate sodium and
- Sulphate
The sulphomethate sodium salt, colistimethate, is available for parenteral administration and by inhalation, while the colistin sulphate salt is not recommended for parenteral use but can be used topically, orally and in susceptibility testing19.
Colistimethate Sodium
From the decline in its use in the early 1970s uptil the mid -1990s, there have been limited studies on the clinical use of colistin or on its pharmacokinetics and pharmacodynamics, as it was never subjected to the drug development process required for compliance with contemporary regulatory requirements.
Recent advances in the development and validation of analytical methods for quantification in biological fluids have enabled new insights into the pharmacokinetics and pharmacodynamics of colistin.
Colistimethate sodium is structurally and pharmacologically related to polymyxin B; however it differs by only one amino acid in the peptide ring of the molecule, and is also considered to be a better drug that polymyxin B on the ground of reduced toxicity19.
Pharmacology
Pharmacodynamics
Mechanism of Action19,20
Colistimethate sodium is a rapid-acting bactericidal agent, with a "detergent-like" mechanism of action. It exhibits two important activities that arise from its interaction with the outer membrane of gram-negative bacteria viz.,Enhancer activity and Anti-endotoxin activity.
Enhancer Activity
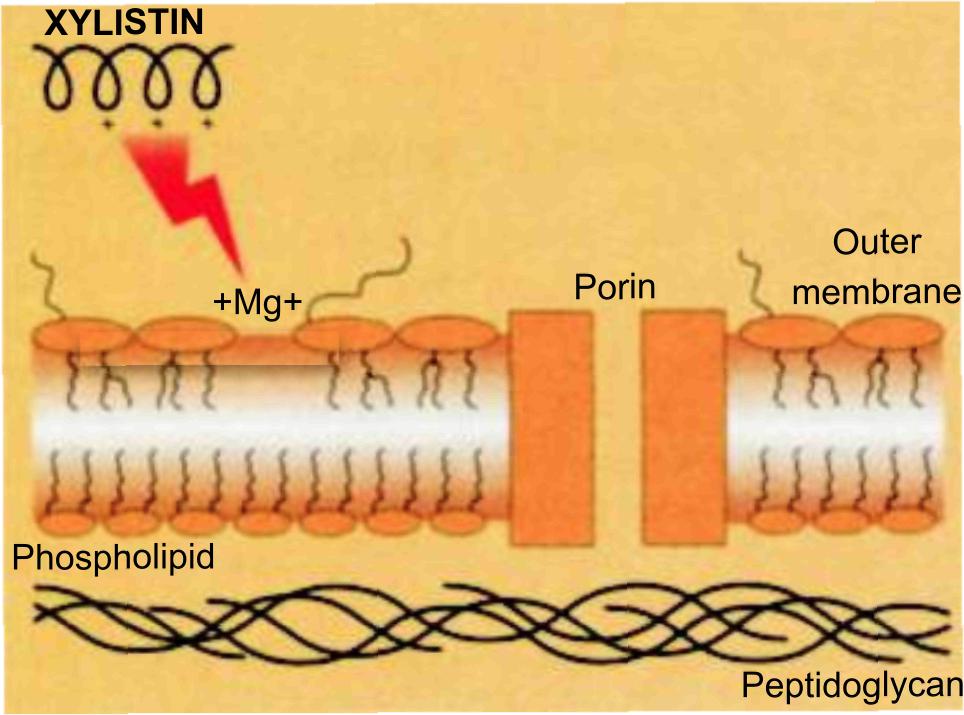
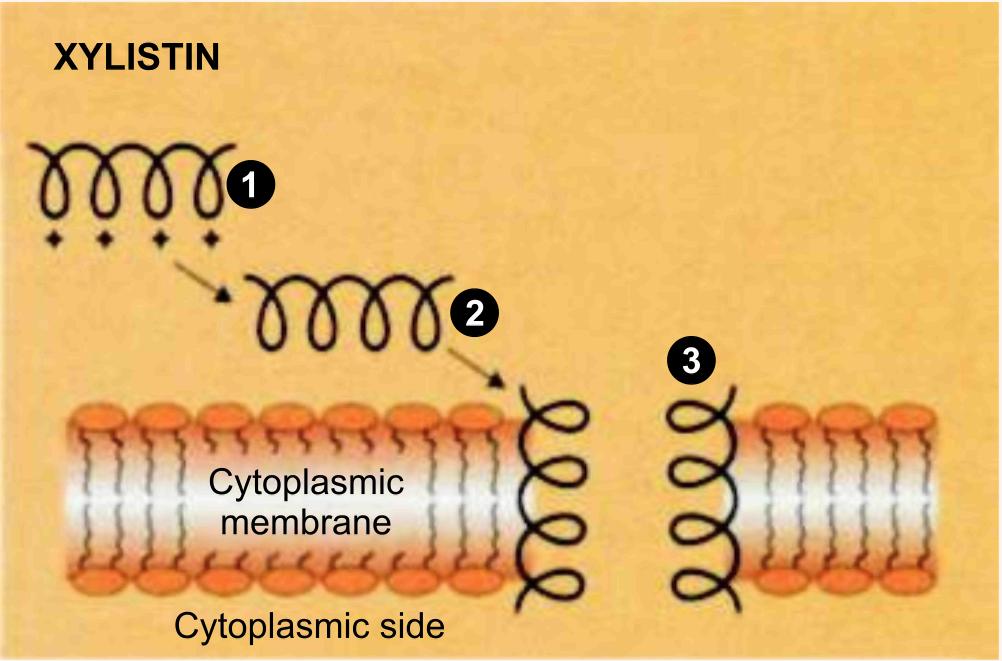
In this pathway, the first step is the binding of colistin to the outer membrane, thereby displacing the calcium and magnesium bridges that stabilize the lipopolysaccharide (LPS).
Because colistin has affinities for LPS that are at least three order of magnitude higher than the divalent cations Ca+2 and Mg+2, they competitively displace these ions and disrupt the outer membrane. The affected membrane is thought to develop transient "cracks" (Figure 14) which permit the uptake of colistin (hence the term "self-promoted uptake"), thereby causing cell death, while also allowing the passage of other antibacterials and thereby exerting synergistic/enhancer activity.
Anti-endotoxin activity
The endotoxin of gram-negative bacteria is the lipid A portion of LPS molecules. Colistin binds and neutralizes the LPS. This property could be beneficial in patients who experience gram-negative bacterial sepsis and endotoxin mediated shock.
Resistance to Colistimethate sodium
There is no cross-resistance seen with colistimethate sodium to other classes of antibacterials. Because of its unique detergent action on the cell membrane, there is virtually no acquired resistance to either colistin or polymyxin B in Pseudomonas aeruginosa or Acinetobacter baumannii.
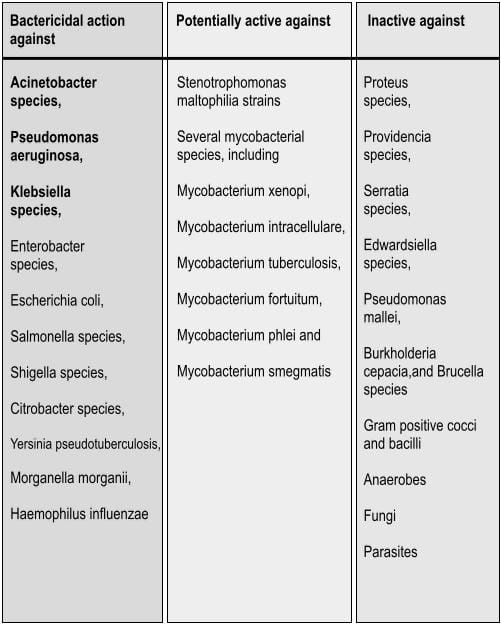
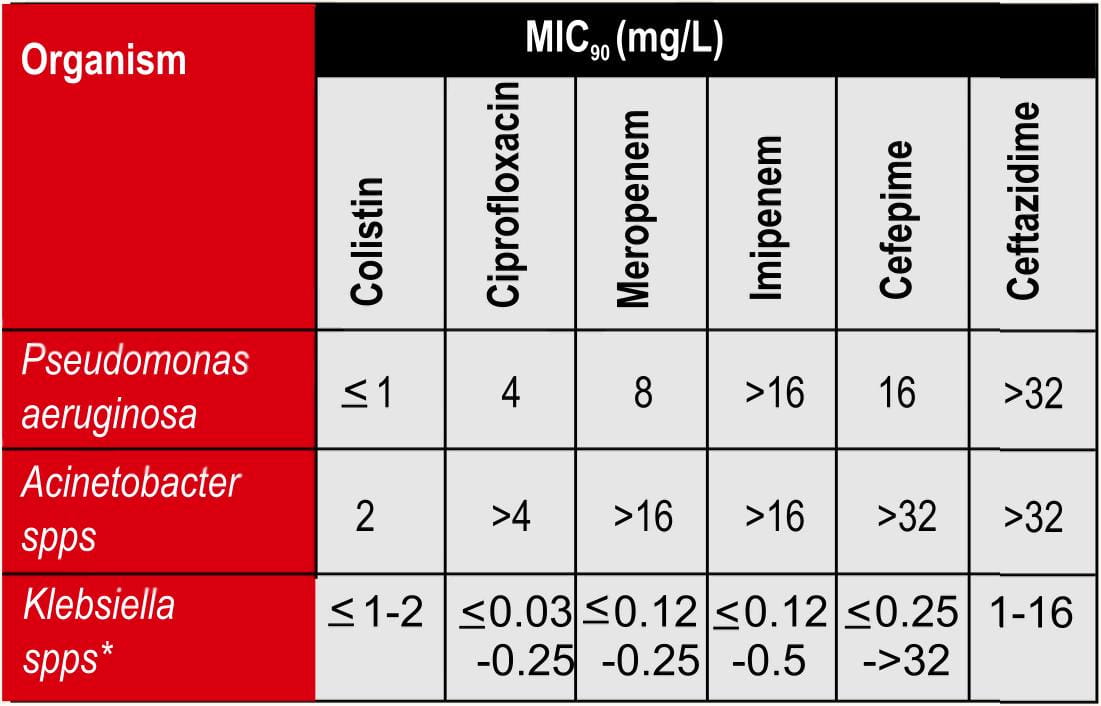
Spectrum of Activity
Colistimethate sodium has a narrow spectrum21 and is selective for gram negative bacteria that have a hydrophobic outer membrane, as mentioned in table 2.
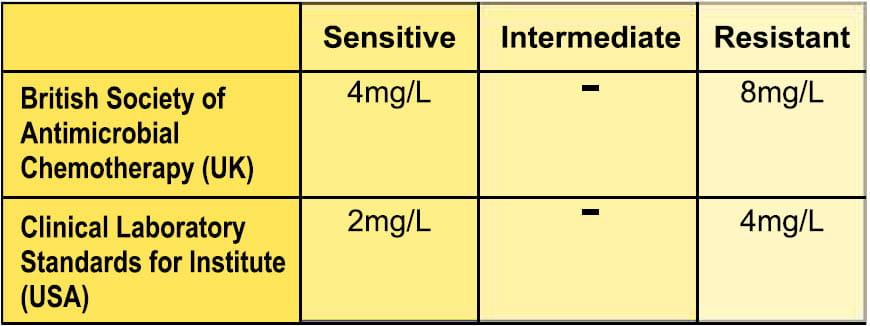
MIC for colistin
Colistimethate sodium being a prodrug of colistin, in vitro susceptibility is performed using colistin sulphate.
With respect to breakpoints (for systemic use only) for susceptibility based on colistin sulphate, the following guidelines have been adopted22,23.
Synergy with other antibacterials
Colistin acts by increasing the permeability of the cell membrane and therefore has been demonstrated to exhibit synergy against gram-negative organisms in combination with other antibacterials, including tetracyclines, chloramphenicol, beta lactams, ciprofloxacin, rifampin and piperacillin.
MDR Gram negative bacteria25
In a study to compare the synergistic activity of colistin with various antibacterial agents like aminoglycosides (amikacin and gentamicin), cephalosporins (ceftazidime and cefotaxime), rifampin and piperacillin, it was seen that piperacillin demonstrated the maximum synergistic activity as shown in the graph below.
The synergy of colistin with piperacillin and rifampin at concentrations attainable in the blood was remarkable and the concentrations used in the above study were 4-8 times lowerthan the MIC forthe individual drugs.
Similarly, in vitro synergistic activity between ceftazidime and colistin was reported against MDR Pseudomonas aeruginosa in a time kill study by Gunderson et al26.
For carbapenem resistant Acinetobacter baumannii, though colistin was found to be bactericidal at concentrations of 4 X MIC and 8 X MIC compared to tigecycline which was found to be bacteriostatic, a combination of colistin / rifampicin and imipenem/sulbactam was synergistic and bactericidal at 1X MIC27.
Pharmacokinetics28,29,30,31
Absorption
Colistimethate sodium must be administered parenterally as it is not absorbed from the gastrointestinal tract, mucous membranes, or intact or denuded skin.
Following IM administration of 150 mg of colistin as colistimethate sodium, peak serum concentrations of antimicrobial activity are attained within 2 hours and average 5-7.5 mcg/mL; serum concentrations of antimicrobial activity may be detectable 12 hours after the dose.
In healthy volunteers given a bolus injection of 150mg (2 MIU approx.) peak serum levels of 18 mg/L were observed 10 minutes after the injection. Following IV administration of colistimethate sodium, peak serum concentrations of antimicrobial activity are higher but decline more rapidly than those achieved with IM administration of the drug.
When given by nebulization, variable absorption has been reported that may depend on the aerosol particle size, nebulizer system and lung status. Studies in healthy volunteers and patients with various infections have reported serum levels from nil to potentially therapeutic concentrations of 4mg/L or more. Therefore, the possibility of systemic absorption should always be borne in mind when treating patients by inhalation.
Distribution
Following IM or IV administration of colistimethate sodium, the drug is widely distributed into body tissues, but only negligible concentrations of antimicrobial activity are attained in synovial, pleural, or pericardial fluids. Animal studies indicate that colistin, like polymyxin B sulphate, reversibly binds to and persists in body tissues such as the liver, kidneys, lungs, heart and muscle. Only minimal concentrations of antimicrobial activity are generally attained in CSF following IM or IV administration of colistimethate sodium in patients with normal or inflamed meninges. However, in a recently published case of meningitis due to MDR Acinetobacter baumannii, the intravenous administration of 1 million international units (MIL) of colistimethate sodium every 6 hours resulted in sufficient CSF penetration (25 % of the serum concentration) to cure the infection. Colistin is reportedly more than 50% bound to serum proteins.
In a study of cystic fibrosis, the steady-state volume of distribution was reported as 0.09 L/kg. Colistin crosses the placenta and is reportedly distributed into milk.
Following IM or IV administration of a single 150-mg dose of colistin as colistimethate sodium in patients with normal renal function, antimicrobial concentrations in the urine reportedly range from 200-25 mcg/mL and 270-15 mcg/mL at 2 hrs and 8 hrs after the dose.
Metabolism
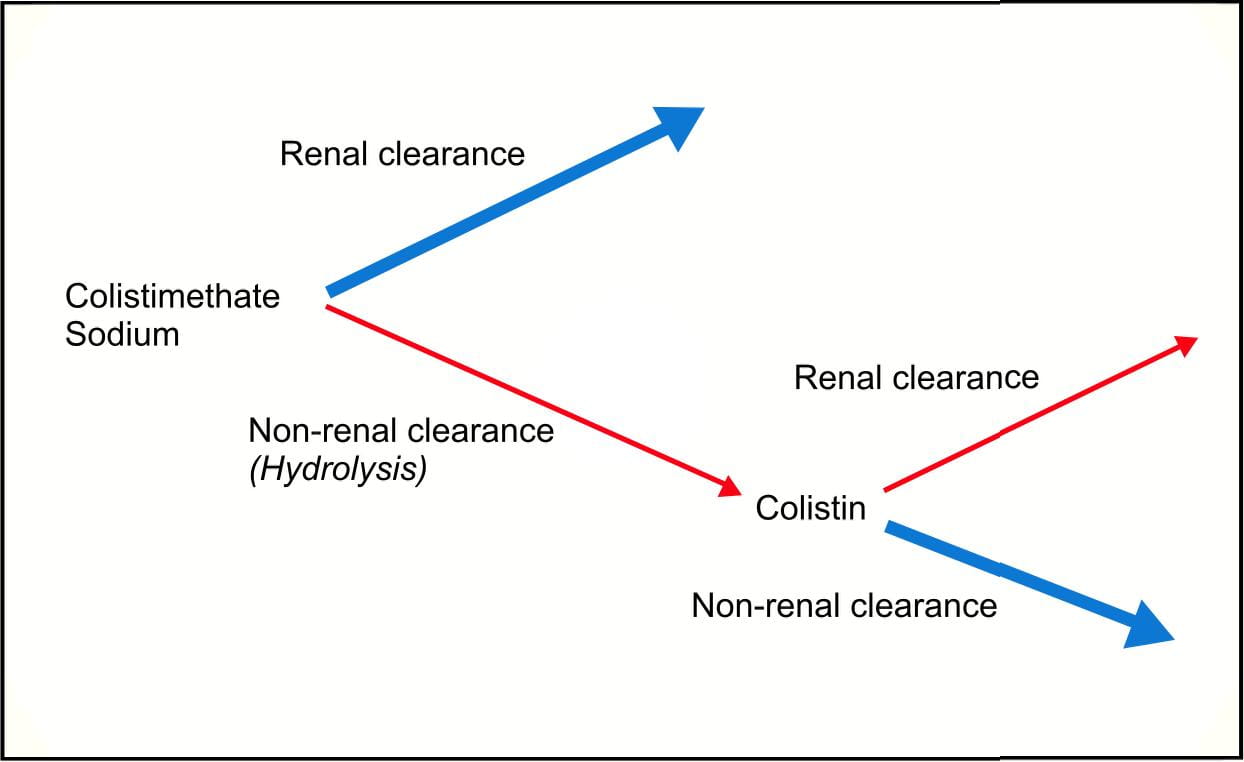
Colistimethate sodium is hydrolyzed in vivo to colistin and possibly other metabolites with fewer substituted amino groups; however, the rate and extent of hydrolysis as well as the specific metabolites and their antibacterial activities have not been determined to date.
Elimination
Colistimethate sodium and its metabolites are excreted mainly by the kidneys through glomerular filtration and urinary excretion. Colistin undergoes extensive renal tubular resorption, contributing to excretion of the drug via nonrenal mechanisms.
Approximately 60% of colistimethate sodium is excreted as unchanged drug in the urine during the first 24 hours after dosing. The plasma half-life of colistimethate sodium after parenteral administration is 1.6-4 hours in adults with normal renal function.
Special populations
Serum concentrations are higher and the half-life is prolonged in patients with impaired renal function, with a potential half-life of 2-3 days in anuric patients. In patients with creatinine clearance less than 20 mL/minute, the half-life of colistin ranges from 10-20 hours.
In patients with impaired renal function, antimicrobial activity in the urine is generally higherthan antimicrobial activity in serum at 24 hours.
It is not known whether colistimethate sodium is removed by haemodialysis or peritoneal dialysis. Serum concentrations of antimicrobial activity following administration of colistimethate sodium appear to decline more rapidly in children than in adults.
Xylistin: Efficacy at a Glance
- Recommended by ATS/IDSA 200532 for carbapenem resistant Acinetobacter VAP.
- Effective therapeutic option against otherwise untreatable Pseudomonas aeruginosa infections.
- Effective as a salvage therapy for carbapenem and pan resistant infections
- Sepsis
- Bloodstream infections
- Urinary infections
- Catheter-related infections
- Bone and Joint infections
- Diabetic Foot infections +/- osteomyelitis33
- As effective and safe as imipenem-cilastatin for MDR Acinetobacter baumannii VAP.
- Safe and effective in paediatrics.
- Aerosolized colistin as an adjunct to intravenous proves to be an effective alternative for difficult to treat VAP.
Efficacy Studies
Since 1999, the clinical reports that have been published on colistimethate sodium conclude that it is an effective therapeutic option for management of MDR infections including carbapenem resistant, with a response rate of 57-73%.
Systemic Treatment
Salvage Therapy for Pan Drug Resistant Pseudomonas aeruginosa Infections34
23 critically ill patients with resistant P. aeruginosa infection, received colistin as a salvage therapy. The most common types of infection were pneumonia (n=18) and intra-abdominal (n=5), while others included bacteraemia and endocarditis.
Colistin was administered for a median of 17 days, and the initial doses were selected on the basis of ideal body weight and estimated creatinine clearance.
Pseudomonas exhibited uniform resistance to all tested antipseudomonal beta-lactams ie: penicillins (piperacillin), cephalosporins (ceftazidime, cefepime), monobactams (aztreonam), carbapenems (imipenem-cilastatin), fluoroquinolones (ciprofloxacin) and aminoglycosides (gentamicin and tobramycin).
Results
A favourable clinical outcome was observed in 61% of patients. Though 3 patients did experience a relapse, they were, however, treated successfully with a second course of colistin therapy.
Conclusion
Despite a high degree of illness severity in a population of predominantly immunocompromised solid-organ transplant recipients, colistin proved to be an important salvage therapeutic option for patients with otherwise untreatable serious Pseudomonas aeruginosa infection.
Salvage Therapy for Carbapenem Resistant Infection35
This was a prospective study which included 75 patients with 78 nosocomial infections caused by MDR Acinetobacter baumannii or Pseudomonas aeruginosa, which were resistant to piperacillin, ceftazidime, imipenem/cilastatin and ciprofloxacin.
Seventy-eight courses of I.V. colistin sulphomethate sodium were given to these patients. The dosage was adjusted according to the renal function of the patient based on the serum creatinine clearance as follows:
- Mean daily dose: 5.5±1.1 MlU/day (range 2-9 MlU/day) and
- Mean duration of therapy: 9.3±3.8 days (range 5-21 days).
Results
Conclusion
Systemic colistimethate sodium therapy therefore remains an effective therapeutic option for the treatement of nosocomial infection cuased by MDR, including carbapenem resistant strains.
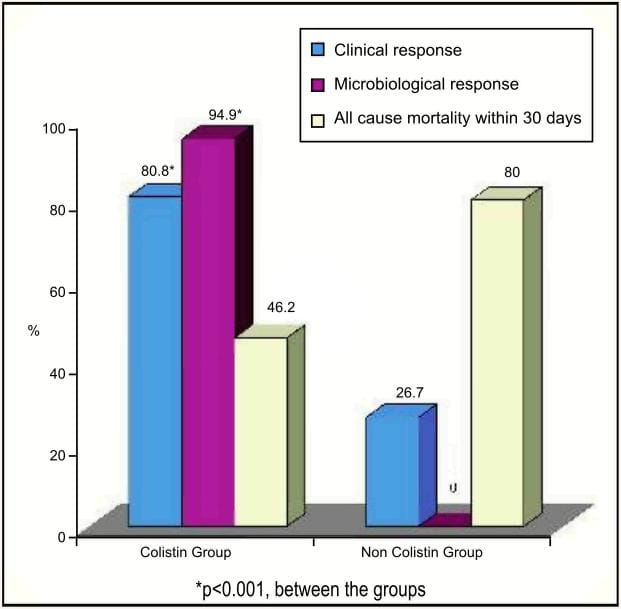
Comparison of Intravenous Colistin for the Treatment of MDR Acinetobacter baumannii and Pseudomonas aeruginosa36Patients who had infections caused due to MDR Acinetobacter baumannii and Pseudomonas aeruginosa received colistimethate sodium at a dosage of 5mg/kg/day in two divided doses.
Clinical response and 30-day mortality were the primary outcomes, while microbiological response and adverse events were the secondary outcomes. Of 71 patients who received colistin, 33 patients received colistin alone, whereas 45 patients received colistin with other antibiotics including vancomycin, aminoglycosides, metronidazole or carbapenems. Presenting infections in the colistin group were: pneumonia, bacteraemia and/or catheter related infection, intra-abdominal infection, urinary tract infection and skin and soft tissue infections.
15 patients who did not wish to join the study, received carbapenems, cefoperazone/sulbactam, cefoperazone/ sulbactam combined with netilmicin, and cefoperazone/sulbactam combined with carbapenem as per the physician's decision.
Results
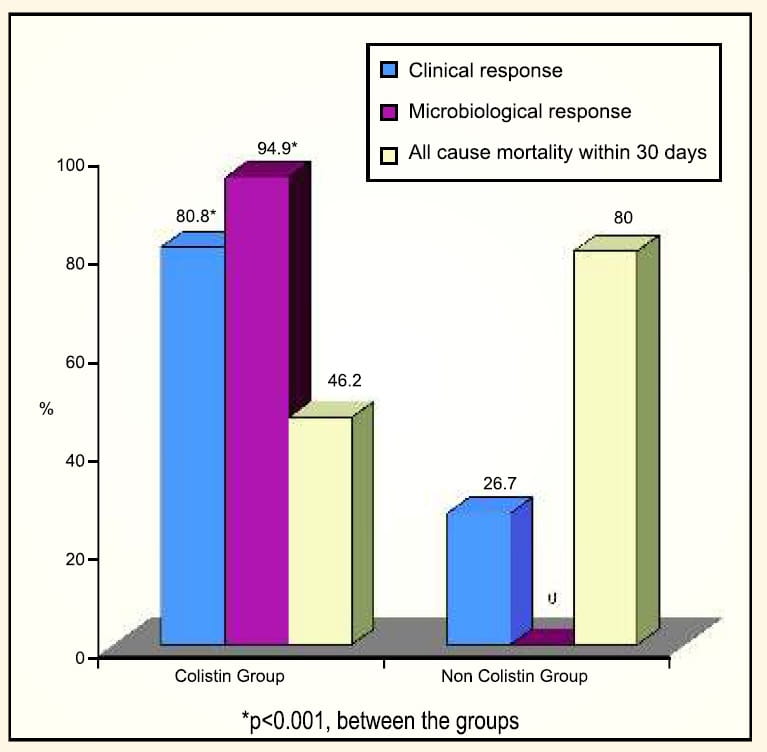
- Overall, good clinical outcome was found in 82.1% of patients treated with colistin, wether used alone or in combination with other antibiotics.
- In this study, when treated with colistin, the overall mortality decreased from 79% (as per a previous study of Acinetobacter baumannii infections in the same hospital) to 46.5%.
- Nephrotoxicity was observed in 30.8% of patients in the colistin group compared to 66.7% in the non-colistin group.
- No neurotoxicity or drug reaction was observed with colistin.
Conclusion
Colistin appears to be safe and effective for the treatment of infections caused by MDR Pseudomonos aeruginosa and Acinetobacter baumanii.
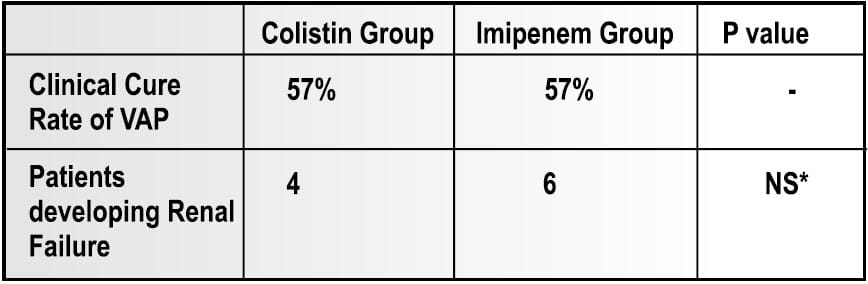
This was a prospective open study of 35 episodes of VAP due to MDR Acinetobacter baumannii. Of 35 patients, 27 had severe sepsis or septic shock attributable to the pulmonary infection.
- 21 patients were treated with IV colistimethate sodium (for Colistin-only susceptible strain)
- 14 patients were treated with imipenem-cilastatin, 2-3 g/d (for Imipenem susceptible strain)
Results
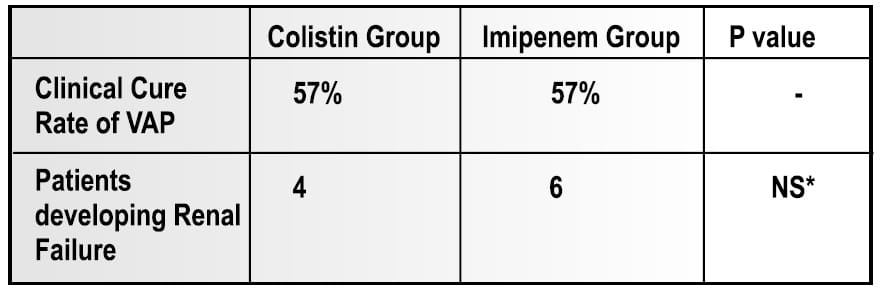
- At the end of the therapy, microbiological eradication of the infection was observed in 66.75% of patients in the colistin group.
- No signs of neuromuscular blockade were seen in any of the colistin group patients.
Conclusion
Intravenous colistin is atleast as effectie and safe as imipene-cilastatin, the conventional treatement of choice for VAP caused by MDR strains od Acinetobacter baumannii. In addition, nephrotoxicity and neurotoxicity were not observed, although these advesrse events have been reported with systemic user of colistin.
This was a retrospective38 review over a period of 11 years, observed in 14 patients at a tertiary paediatric burn center. Out of 14 patients, 11 patients and 2 patients were treated with colistin for pan-resistant Pseudomonas aeruginosa and Acinetobacterbaumannii with 16 courses of colistin, respectively.
Mean number of days of colistin therapy was 15.8 ± 13.3 and mean dosage (mg/kg/d) was 4.74 ± 0.73, administered in three to four divided doses.
Results
- 78.6% (11/14) of patients demonstrated prompt improvement in their clinical status, survived, and were eventually discharged home.
- Although two children developed significant increase in serum creatinine concentration, no child required renal replacement therapy.
- No development of neurological complications attributable to colistin was seen.
- Overall mortality was 14.3% (2/14); both deaths were attributed to sepsis.
Conclusion
Colistimethate soduim should be dispensed with great caution, but it appears to have an acceptable safety profile in children and may be used in select cases of infection with highly resistant gram-negative organisms.
Aerosolized colistimethate sodium as an adjunctive treatment for MDR Gram-negative VAP
Though aerosolized colistimethate sodium has been used mainly in patients with cystic fibrosis, reports of a few published experiences indicate that aerosolized colistin may be a beneficial and safe additional therapeutic intervention for the management of difficult-to-treatVAP.
One of these was a prospective study39 which enrolled 60 critically ill patients, with mean APACHE II score of 16.7 and a very high severity of illness. Therapy with aerosolized colistimethate sodium in combination with other antibacterial agents was initiated only when VAP was documented and the responsible gram-negative pathogen was MDR, but sensitive to colistin.
Acinetobacter baumannii, Pseudomonas aeruginosa and Klebsiella pneumoniae were the common pathogens, half of which were susceptible only to colistin.
The daily dosage of aerosolized colistimethate sodium was 3 MIU divided into three doses, while the daily dosage of IV colistimethate sodium was 9 MIU divided into three doses in patients with normal renal function. The selection of the second antibiotic was based on the susceptibility test and MICs.
Bacteriological and clinical response ofVAP was associated with improvement of chest X-ray and arterial blood gas, and normalization of white blood count, C-reactive protein and procalcitonin.
Conclusion
Aeroslized colistimethate sodium may be considered as an adjunctive to intraveneuos treatement in patients with VAP due to MDR gram negative bacteria susceptible to colistin.
Kwa AL et al40 studied, nebulized colistin in 21 patients with pneumonia caused by polymyxin-only susceptible Acinetobacter baumannii and Pseudomonas aeruginosa. Even though none of the patients in this study received intravenous colistin and the other antibiotics were inactive in vitro against the isolated pathogens, overall clinical and microbiological response rates were 57.1% and 85.7%, respectively.
Xylistin: Safety Profile and Dosage at a Glance
- Adverse events are not as frequent as previously reported and feared of, on the basis of avoidance of concurrent use of nephrotoxic and neurotoxic drugs and dose modification in renally compromised patients.
- No serious toxicity reported even on prolonged (more than 4 weeks) administration.
- Recommended upper dose limit is 6 million lU/day, however in 9 million lU/day is found to be safe and effective.
- In obese patients, dosage should be based on ideal body weight.
- Quick diuresis with IV mannitol and exchange transfusion may enhance renal clearance in setting of overdose.
- Solution forsingle use only.
Safety and Tolerability
Several reports during the early years of use of the polymyxins, especially during 1960-1969 gave an impression to the medical community that polymyxins are very toxic (nephrotoxic and neurotoxic). However, the recent experience of several clinicians worldwide on using this medication failed to verify the older reports about the toxicity of colistin. It is very likely that the toxicity observed with the earlier clinical use was partly due to the lack of understanding of the pharmacokinetics, pharmacodynamics and toxicodynamics of polymyxins. Of significant interest are recent comparative studies in cystic fibrosis patients41 which demonstrated that parenteral colistimethate sodium may be less nephrotoxic than aminoglycosides. This finding is remarkable; given that it was the aminoglycosides that caused the polymyxins to go out of favour, several decades ago.
In critically ill patients, recent studies42,37 have reported a relatively low incidence of renal function impairment with parenteral administration of colistimethate sodium.
Study : No Serious Toxicity on Prolonged Administration of IV Colistimethate Sodium
Falagas et al43 investigated the adverse effects of prolonged courses ie. more than 4 weeks (one course) of intravenous colistin in 17 patients, for the treatment of MDR gram-negative infections. It was an observational retrospective study. Here 2 patients received two prolonged courses of intravenous colistin each, with a colistin-free period of more than 1 month between the courses.
The patients were thus given 19 courses of prolonged intravenous colistin.
- Mean duration of administration: (± SD) 43.4 (± 14.6) days
- Mean daily dosage: (± SD) 4.4 (± 2.1) MIU
- Mean cumulative dosage: (±SD) 190.4 (±91.0) MIU
Serum creatinine, blood urea, liver function tests and symptoms and signs of neurotoxicity were the main outcomes studied.
For patients with impaired renal function, dosage adjustments were done based on the following protocol:
- If serum creatinine level was 1.3-1.5 mg/dL, 1.6-2.5 mg/dL or2.6 mg/dL, the dosage of colistimethate sodium administered was 2 MIU (160 mg) every 12 hours, 24 hours, or 36 hours, respectively.
- Patients who were on dialysis treatment received 1 MIU (80 mg) of colistimethate sodium after dialysis.
Results
No significant deterioration of renal function was found in patients with baseline normal serum creatinine value.
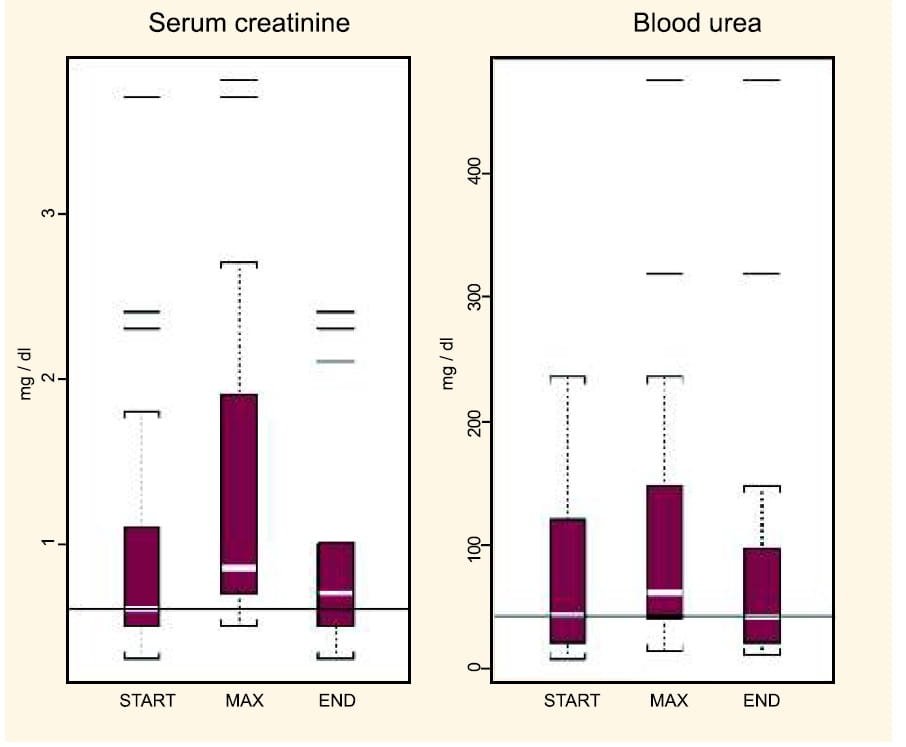
- Though there was a slight increase in the serum creatinine values at the end of treatment (0.1 mg/dL increase in the median creatinine values from the start to end of colistin administration), this cannot be attributed to the prolonged colistin administration only. This is because other factors such as concomitantly administered potential nephrotoxic agents (like vancomycin and aminoglycosides) and sepsis perse may have had an effect.
- In the group of patients with pre-existing chronic renal dysfunction (not on haemodialysis), the administration of the medication for a long duration did not further influence their renal function.
- Apnoea or other evidence of neuromuscular blockade was not observed in any of the 17 patients who received prolonged treatment with colistin.
Conclusion
Intrvenous colistin seems to be a safe therapeutic intervention for serious infections due to MDR grsm-negative bacteria even when it is administered for prolonged duration(more than 4 weeks)
Difference Between the Old and Recent Toxicity Reports
In a systematic review of the evidence from old and recent studies it has been shown that polymyxins have less toxicity than previously reported44.
Possible explanations for the observed discrepancy are as follows: -
- During 1970, colistimethate sodium available for intramuscular administration use was provided in vials containing a colistin base and a local anaesthetic, dibucaine hydrochloride, which could have potentiated the neurotoxic effect of colistimethate sodium. The same was used intravenously until a new formulation was prepared.
- The dosages of polymyxins used in most of the studies published in the old literature were considerably higher compared to the recommended dosages administered nowadays. In one study45 of 14 patients, doses of 2600 mg of colistin were used, whereas the recommended maximum daily dose is 480 mg and 800 mg in the UK and USA, respectively. This suggests that several reported cases of polymyxin-induced toxicity were associated with overdosage.
- Dosage adjustment of polymyxins in the presence of impaired renal function and prompt discontinuation of polymyxins after development of early signs of theirtoxicity were not always performed in a timely fashion.
- Further there were also other factors, such as the co-administration of medications with potential toxicity, like anaesthetic drugs and muscle relaxants, which perhaps contributed to the toxicity.
The already reported experience regarding the toxicity of polymyxins in the old literature has led to a more correct use of these antibiotics by physicians nowadays. In addition, the avoidance of co-administration of potential nephrotoxic and/or neurotoxic agents with polymyxins, as well as the development of critical care supplies, may also explain the observed differences.
Management and Prevention of Adverse Effects
NephrotoxicityRisk Factors
Nephrotoxicity resulting from the use of colistimethate sodium appears to be less compared with that associated with polymyxin B. Concomitant administration of potential nephrotoxic agents, such as diuretics and some antimicrobial agents, increases the likelihood of development of renal adverse effects.
Signs
Proteinuria, haematuria and casts in the urine may occur, but rising blood urea and serum creatinine values are the most invariable features. These usually occur within the first 4 days of the therapy. Acute tubular necrosis may result and this may not be preceded by progressive renal function impairment44.
Treatment
Nephrotoxicity is dose dependent and hence the dose can be decreased at the onset of renal dysfunction. The effects are usually reversible although often not immediately. Renal function usually returns to baseline in 3-9 weeks.
When primary signs of renal dysfunction occur, the following measures need to be taken:
- early discontinuation of colistimethate sodium
- meticulous supportive care, including close monitoring of fluid intake and output
- frequent determinations of electrolytes and
- appropriate management to maintain balance of fluids and electrolytes.
In addition to the above measures, quick diuresis by intravenously administered mannitol and exchange transfusion has been proposed to enhance renal clearance of the drug and, therefore, reduce serum drug levels.
The influence of haemodialysis and peritoneal dialysis in decreasing serum levels of polymyxins has not been clarified. Old reports suggested that the amount of drug that is removed from blood by these two methods is relatively small. Approximately 1 mg/hr of colistin is removed from the body by peritoneal dialysis, with an average of 16% of the total dose removed during a 2-hour peritoneal dialysis session.
Thus, in cases of polymyxin induced renal failure, both therapeutic approaches can be used, not to decrease serum drug levels but in order to manage renal complications21.
In a study conducted by Li et al46, when colistimethate sodium 150 milligrams every 48 hours was administered intravenously to a 53-year-old woman (total body weight, 100 kilograms (kg); ideal body weight, 61 kg) with bowel perforation leading to multiple organ failure which required continuous venovenous haemodiafiltration (CVVHDF), both colistimethate sodium and colistin were cleared by CVVHDF. From 0- 8 hours after dosing, 20.3% and 6.88% of the dose was recovered in the dialysate as colistimethate sodium and colistin, respectively.
Risk Factors
Hypoxia and co-administration of polymyxins with muscle-relaxants, narcotics, sedatives, anaesthetic drugs or corticosteroids may have potential for the development of neurotoxicity. Patients with impaired renal function or myasthenia gravis are at higher risk of developing neuromuscular blockade and respiratory paralysis.
Treatment
Mild neurological manifestations of polymyxins usually subside after prompt cessation of the treatment. In the presence of neuromuscular blockade, immediate discontinuation of polymyxins and other neurotoxic agents is the first-line approach. Further management consists of mechanical respiratory support if apnoea has developed. The intravenous administration of calcium and cholinesterase inhibitors, such as neostigmine and edrophonium, has led to conflicting results. Haemodialysis is indicated only in patients with co-existing acute renal failure.
Prevention of Adverse Events44
Early and correct dose adjustment of polymyxins, in the presence of impaired renal function, frequent urinalyses and serum urea or creatinine measurements, close daily monitoring of urinary output and of neurological status, and the avoidance of concurrent administration of other agents with known nephrotoxicity or neurotoxicity may help prevent the development of adverse effects. Bronchoconstriction usually responds to treatment with bronchodilators; therefore, pre-treatment of patients receiving inhaled colistimethate sodium with these medications could prevent the occurrence of bronchoconstriction.
Indications, Dosage and Precautions
Xylistin is indicated22 in the treatment of the following infections, where sensitivity testing suggests that they are caused by susceptible bacteria:
- Intravenous administration for the treatment of some serious infections caused by Gram-negative bacteria, including those of the lower respiratory tract and urinary tract, when more commonly used systemic antibacterial agents may be contraindicated or may be ineffective because of bacterial resistance.
- Treatment via inhalation, of Pseudomonas aeruginosa lung infection in patients with cystic fibrosis.
Dosage and Method of Administration
The dose is determined by the severity and type of infection, and the age, weight and renal function of the patient22.
Xylistin can be given as a 50 mL intravenous infusion over a period of 30 minutes. Patients with a totally implantable venous access device (TIVAD, tube fitted inside a vein to allow drug injections) in place may tolerate a bolus injection of up to2 MIU in 10 mLgiven overa minimum of 5 minutes.
Up to 60kg
50,000 lU/kg/day to a maximum of 75,000 lU/kg/day. The total daily dose should be divided into three doses given at approximately 8-hour intervals.
Over 60kg
1-2 million units three times a day. The maximum dose is 6 million units in 24 hours.
Should clinical or bacteriological response be slow, the dose may be increased as indicated by the patient's condition.
A minimum of 5 days of treatment is generally recommended. For the treatment of respiratory exacerbations in cystic fibrosis patients, treatment should be continued for up to 12 days.
Anomalous distribution in patients with cystic fibrosis may require higher doses in orderto maintain therapeutic serum levels.
Renal Impairment: In moderate to severe renal impairment, excretion of colistimethate sodium is delayed. Therefore, the dose and dose interval should be adjusted in order to prevent accumulation. The table below is a guide to dose regimen modifications in patients of 60 kg bodyweight or greater. It is emphasized that further adjustments may have to be made based on blood levels and evidence of toxicity.
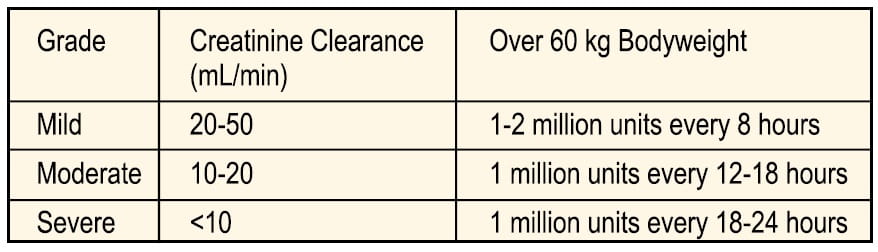
Serum level estimations are recommended especially in neonates, renally impaired and cystic fibrosis patients. Levels of 10-15 mg/L (approximately 125-200 units/mL) colistimethate sodium should be adequate for most infections.
Dosing recommendations for patients undergoing renal replacement therapy has not been well established. However, because of poor clearance, colistimethate sodium should be dosed at 2 mg/kg/d during peritoneal dialysis21. There are no available data about the need, if any, for dosage modification in patients with liver failure. In obese patients, dosage should be based on ideal body weight.
The normal adult dose of 2 million units should be dissolved in 10-50 mL of 0.9% sodium chloride intravenous infusion or water for injection to form a clear solution. The solution is for single use only and any remaining solution should be discarded.
For local treatment of lower respiratory tract infections, colistin powder is dissolved in 2-4 mL of water for injection or 0.9% sodium chloride intravenous infusion for use in a nebuliser attached to an air/oxygen supply.
In small, uncontrolled clinical trials, doses of 500,000 units twice daily up to 2 million units three times daily have been found to be safe and effective in patients with cystic fibrosis.
The following recommended doses are for guidance only and should be adjusted according to clinical response:
Children <2 years: 500,000-1 million units twice daily
Children >2 years and Adults: 1-2 million units twice daily
The required amount of powder is dissolved, preferably, in 2-4 mL of 0.9% sodium chloride solution and poured into the nebulizer. Alternatively, water for injection may be used. The solution will be slightly hazy and may froth if shaken. Usually jet or ultrasonic nebulisers are preferred for antibiotic delivery. These should produce the majority of their output in the respirable particle diameter range of 0.5-5.0 microns when used with a suitable compressor. The instructions of the manufacturers should be followed for the operation and care of the nebulizer and compressor.
The output from the nebulizer may be vented into the open air or a filter may be fitted. Nebulization should take place in a well-ventilated room.
The solution is for single use only and any remaining solution should be discarded. Colistimethate is hydrolysed into two active components, colistin A (polymyxin E1) and colistin B (polymyxin E2). In animal studies, polymyxin E1 has been shown to cause localized inflammation of the airway epithelia and eosinophilic infiltration. Storing colistimethate aqueous solution for longer than 24 hours increases colistin concentration and increases the potential for lung injury.
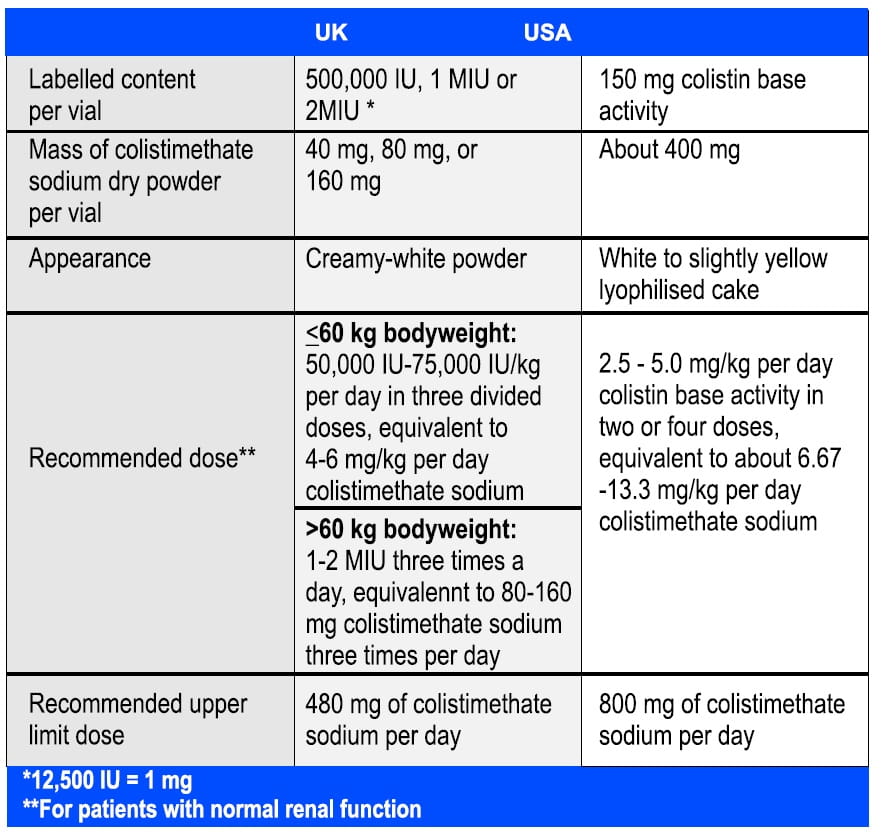
The table below shows the comparison of the two major types of colistimethate sodium parenteral products in the UK and USA31
Contraindications
Xylistin is contraindicated in patients with known hypersensitivity to colistimethate sodium (colistin) or to polymyxin B. Colistimethate sodium is known to reduce the amount of acetylcholine released from the pre-synaptic neuromuscular junction. Hence, it should not be used in patients with myasthenia gravis.
Warnings and Precautions
Xylistin must be used with extreme caution in patients with porphyria. Nephrotoxicity or neurotoxicity may occur if the recommended parenteral dose is exceeded. Use with caution in renal impairment, as colistimethate sodium is renally excreted. It is advisable to assess baseline renal function and to monitor it during treatment. Serum colistimethate sodium concentrations should be monitored.
Transient neurological disturbances like circumoral paraesthesia or numbness, tingling or formication of the extremities, generalized pruritus, vertigo, dizziness, and slurring of speech may occur. Hence, patients should be warned not to drive vehicles or use hazardous machinery while on therapy. However, while therapy need not be discontinued, such patients should be observed with particular care.
Pseudomembranous colitis has been reported with nearly all antimicrobial agents, and may range in severity from mild to life threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhoea subsequent to the administration of antibacterial agents.
Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against Clostridium difficile colitis.
Concomitant use of colistimethate sodium with other medicinal products of neurotoxic and/or nephrotoxic potential should be avoided. These include the aminoglycoside antibiotics such as gentamicin, amikacin, netilmicin and tobramycin. There may be an increased risk of nephrotoxicity if given concomitantly with cephalosporin antibiotics.
Neuromuscular blocking drugs and ether should be used with extreme caution in patients receiving colistimethate sodium.
Xylistin: Place in Therapy
After being almost forgotten for more than 20 years, the recent increase in the use of colistimethate sodium is a consequence of emerging carbapenem and pan drug resistant Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacteriaceae (particularly in ICUs), the resultant high mortality rate, and the limited number of therapeutic options available.
Colistimethate sodium, though an older drug, seems to be a novel antibiotic option for patients with otherwise untreatable serious infections:
- Lower respiratory tract
- Urinary tract
- Sepsis
- Fixation device related orthopaedic infections
- Multiresistant central nervous system infections (intrathecal, intraventricular colistin)
- Catheter related infections
It has rapid, concentration-dependent bactericidal activity against Gramnegative pathogens and exhibits considerable post-antibiotic effect. As a combination therapy it is found to be effective against multidrug and pandrug resistant (resistant to all antibiotic classes) Gram-negative bacteria like:
- Acinetobacter spps
- Pseudomonas aeruginosa
- Klebsiella spps
- Enterobacteriaceae
For the Use of a Registered Medical Practitioner or a Hospital or a Laboratory Only
Colistimethate Sodium Powder For Solution For Injection, Infusion 1 Million IU
Xylistin: Prescribing Information
Composition
Each vial contains:
1 Million International Units (1 MIU) Colistimethate Sodium
Dosage Form
Powder for solution for injection, infusionPharmacology
Pharmacodynamics
Mode of Action
Colistimethate sodium is a cyclic polypeptide antibiotic derived from Bacillus polymyxa var. colistinus and belongs to the polymyxin group. The polymyxin antibiotics are cationic surface-active agents that work by damaging the cell membrane. The resulting physiological effects are lethal to the bacterium. Polymyxins are selective for Gram-negative bacteria that have a hydrophobic outer membrane.
Microbiology
Commonly susceptible species
Acinetobacter species*
Citrobacter species
Escherichia coli
Haemophilus influenzae
Pseudomonas aeruginosa
Species for which acquired resistance may be a problem
Enterobacter species
Klebsiella species
Inherently resistant organisms
Brucella species
Burkholderia cepacia and related species
Neisseria species
Proteus species
Providencia species
Serratia species
Anaerobes and
All Gram-positive organisms.
*ln vitro results may not correlate with clinical responses in the case of Acinetobacter species.
When necessary, expert advice should be sought when the local prevalence of resistance is such that the utility of the agent in at least some types of infections is questionable.
When necessary, expert advice should be sought when the local prevalence of resistance is such that the utility of the agent in at least some types of infections is questionable.
Cross-resistance
Cross-resistance between colistimethate sodium and polymyxin B would be expected. Since the mechanism of action of the polymyxins is different from that of other antibiotics, resistance to colistin and polymyxin by the above mechanism alone would not be expected to result in resistance to other drug classes.
Pharmacokinetics
Absorption
Absorption from the gastrointestinal tract does not occur to any appreciable extent in the normal individual.
When given by nebulisation, variable absorption has been reported that may depend on the aerosol particle size, nebuliser system and lung status. Studies in healthy volunteers and patients with various infections have reported serum levels from nil to potentially therapeutic concentrations of 4 mg/L or more. Therefore, the possibility of systemic absorption should always be borne in mind when treating patients by inhalation.
In healthy volunteers given a bolus injection of 150 mg (2 million units approximately), peak serum levels of 18 mg/L are observed 10 minutes after injection.
In patients with cystic fibrosis, after administration of 7.5 mg/kg/day in divided doses given as 30-minute intravenous infusions to steady-state, the Cmax was determined to be 23±6 mg/L and the Cmin at 8 hours was 4.5±4 mg/L. 2 million units, when administered every 8 hours in similar patients for 12 days, the Cmax achieved was 12.9 mg/L (5.7-29.6 mg/L) and the Cmin was 2.76 mg/L (1.0-6.2 mg/L).
Distribution
Protein binding is low. Polymyxins persist in the liver, kidneys, brain, heart, and muscle. The steady-state volume of distribution in cystic fibrosis patients is 0.09 L/kg.
Biotransformation
Colistimethate sodium undergoes conversion to its base in vivo. Approximately 80% of the dose is recoverable unchanged in the urine. There is no biliary excretion and any remaining drug is believed to be inactivated in the tissues.
Elimination
The main route of elimination after parenteral administration is by renal excretion with 40% of a parenteral dose recovered in the urine within 8 hours and around 80% in 24 hours. Because colistimethate sodium is largely excreted in the urine, dose reduction is required in renal impairment to prevent accumulation (refer under Dosage and Method of Administration).
After intravenous administration to healthy adults, the elimination half-life is around 1.5 hours. In a study in cystic fibrosis patients given a single 30-minute intravenous infusion, the elimination half-life was 3.4±1.4 hours. The elimination of colistimethate sodium following inhalation has not been studied.
Colistimethate sodium kinetics appear to be similar in children and adults, including the elderly, provided renal function is normal. Limited data are available on use in neonates which suggest kinetics are similar to children and adults but the possibility of higher peak serum levels and prolonged half-life in these patients should be considered and serum levels monitored.
Indications
Xylistin is indicated in the treatment of the following infections, where sensitivity testing suggests that they are caused by susceptible bacteria:
- Intravenous administration for the treatment of some serious infections caused by Gram-negative bacteria, including those of the lower respiratory tract and urinary tract, when more commonly used systemic antibacterial agents may be contraindicated or may be ineffective because of bacterial resistance.
- Treatment by inhalation of Pseudomonas aeruginosa lung infection in patients with cystic fibrosis.
Dosage and Method of Administration
Systemic Treatment
Xylistin can be given as a 50 mL intravenous infusion over a period of 30 minutes. Patients with a totally implantable venous access device (TIVAD) in place may tolerate a bolus injection of up to 2 million units in 10 mL given over a minimum of 5 minutes (see Reconstitution for Parenteral Administration).
The dose is determined by the severity and type of infection and the age, weight and renal function of the patient. Should clinical or bacteriological response be slow the dose may be increased as indicated by the patient's condition.
A minimum of 5 days treatment is generally recommended. For the treatment of respiratory exacerbations in cystic fibrosis patients, treatment should be continued for up to 12 days.
Children and Adults (Including the Elderly)
Up to 60kg: 50,000 units/kg/day to a maximum of 75,000 units/kg/day. The total daily dose should be divided into three doses given at approximately 8-hour intervals.
Over 60kg: 1-2 million units three times a day. The maximum dose is 6 million units in 24 hours.
Anomalous distribution in patients with cystic fibrosis may require higher doses in orderto maintain therapeutic serum levels.
Renal Impairment: In moderate to severe renal impairment, excretion of colistimethate sodium is delayed. Therefore, the dose and dose interval should be adjusted in order to prevent accumulation. The table below is a guide to dose regimen modifications in patients of 60 kg bodyweight or greater. It is emphasized that further adjustments may have to be made based on blood levels and evidence of toxicity.
Serum level estimations are recommended especially in renal impairment, neonates and cystic fibrosis patients. Levels of 10-15 mg/L (approximately 125-200 units/mL) colistimethate sodium should be adequate for most infections.
Reconstitution for Parenteral Administration
The normal adult dose of 2 million units should be dissolved in 10-50 mL of 0.9% sodium chloride intravenous infusion or water for injection to form a clear solution. The solution is for single use only and any remaining solution should be discarded.
For local treatment of lower respiratory tract infections, Xylistin powder is dissolved in 2-4 mL of water for injection or 0.9% sodium chloride intravenous infusion for use in a nebuliser attached to an air/oxygen supply (see Reconstitution for Inhalation).
In small, uncontrolled clinical trials, doses of from 500,000 units twice daily up to 2 million units three times daily have been found to be safe and effective in patients with cystic fibrosis.
The following recommended doses are for guidance only and should be adjusted according to clinical response:
Children <2 years: 500,000-1 million units twice daily
Children>2 years and Adults: 1-2 million units twice daily
The required amount of powder is dissolved, preferably, in 2-4 mL of 0.9% sodium chloride solution and poured into the nebuliser. Alternatively, water for injection may be used. The solution will be slightly hazy and may froth if shaken. Usually jet or ultrasonic nebulisers are preferred for antibiotic delivery. These should produce the majority of their output in the respirable particle diameter range of 0.5-5.0 microns when used with a suitable compressor. The instructions of the manufacturers should be followed for the operation and care of the nebuliser and compressor.
The output from the nebuliser may be vented to the open air or a filter may be fitted. Nebulisation should take place in a well-ventilated room.
The solution is for single use only and any remaining solution should be discarded.
Contraindications
Xylistin is contraindicated in patients with known hypersensitivity to colistimethate sodium (colistin) or to polymyxin B and in patients with myasthenia gravis.
Warnings and Precautions
Use with extreme caution in patients with porphyria.
Nephrotoxicity or neurotoxicity may occur if the recommended parenteral dose is exceeded.
Use with caution in renal impairment (see Dosage and Method of Administration) as colistimethate sodium is renally excreted. It is advisable to assess baseline renal function and to monitor during treatment. Serum colistimethate sodium concentrations should be monitored.
Bronchospasm may occur on inhalation of antibiotics. This may be prevented or treated with appropriate use of beta2-agonists. If troublesome, treatment should be withdrawn.
Concomitant use of colistimethate sodium with other medicinal products of neurotoxic and/or nephrotoxic potential should be avoided. These include the aminoglycoside antibiotics such as gentamicin, amikacin, netilmicin and tobramycin. There may be an increased risk of nephrotoxicity if given concomitantly with cephalosporin antibiotics.
Neuromuscular blocking drugs and ether should be used with extreme caution in patients receiving colistimethate sodium.
Use with caution in renal impairment as colistimethate sodium is renally excreted and please refer under Dosage and Method of Administration.
No data available.
There are no adequate data from the use of colistimethate sodium in pregnant women. Single dose studies in human pregnancy show that colistimethate sodium crosses the placental barrier and hence should be used during pregnancy only if the potential benefit justifies the potential risk to the foetus.
Colistimethate sodium is secreted in breast milk. Colistimethate sodium should be administered to breastfeeding women only when clearly needed.
Please refer under Pharmacokinetics and Dosage and Method of Administration.
Elderly patients are more likely to have decreased renal function, hence care should be taken in dose selection and it may be useful to monitor renal function.
Undesirable Effects
Systemic TreatmentThe likelihood of adverse events may be related to the age, renal function and condition of the patient.
In cystic fibrosis patients, neurological events have been reported in up to 27% of patients. These are generally mild and resolve during or shortly after treatment. Neurotoxicity may be associated with overdose, failure to reduce the dose in patients with renal insufficiency and concomitant use of either neuromuscular blocking drugs or other drugs with similar neurological effects. Reducing the dose may alleviate symptoms. Effects may include apnoea, transient sensory disturbances (such as facial paraesthesia and vertigo) and, rarely, vasomotor instability, slurred speech, visual disturbances, confusion, or psychosis.
Adverse effects on renal function have been reported, usually following use of higher than recommended doses in patients with normal renal function, or failure to reduce the dosage in patients with renal impairment, or during concomitant use of other nephrotoxic drugs. The effects are usually reversible on discontinuation of therapy.
In cystic fibrosis patients treated within the recommended dosage limits, nephrotoxicity appears to be rare (less than 1%). In seriously ill, hospitalized, non-cystic fibrosis patients, signs of nephrotoxicity have been reported in approximately 20% of patients.
Hypersensitivity reactions, including skin rash and drug fever, have been reported. If these occur, treatment should be withdrawn. Local irritation at the site of injection may occur.
Inhalation may induce coughing or bronchospasm.
Sore throat or mouth has been reported and may be due to Candida albicans infection or hypersensitivity. Skin rash may also indicate hypersensitivity; if this occurs, treatment should be withdrawn.
Overdosage
Overdose can result in neuromuscular blockade that can lead to muscular weakness, apnoea, and possible respiratory arrest. Overdose can also cause acute renal failure characterized by decreased urine output and increased serum concentrations of BUN and creatinine.
There is no specific antidote, so overdose should be managed by supportive treatment. Measures to increase the rate of elimination of colistin, e.g., mannitol diuresis, prolonged haemodialysis or peritoneal dialysis may be tried, but effectiveness is unknown.
Incompatibilities
Mixing drugs in infusions, injections and nebuliser solutions involving colistimethate sodium should be avoided.
The addition of other antibiotics such as erythromycin, tetracycline, and cephalothin to solutions of Xylistin may lead to precipitation.
Storage and Handling Instructions
Do not store above 25°C. Keep the vials in the outer carton.
Solutions for infusion or injection
Chemical and physical in-use stability for 28 days at 4°C has been demonstrated.
From a microbiological point of view, solutions should be used immediately. If not used immediately in-use storage times and conditions prior to use are the responsibility of the user. They would normally be no longer than 24 hours at 2 to 8°C, unless reconstituted and diluted under controlled and validated aseptic conditions.
Solutions for nebulisation have similar in-use stability and should be treated as above. Patients self-treating with nebulised antibiotic should be advised to use solutions immediately after preparation. If this is not possible, solutions should not be stored for longer than 24hrs in a refrigerator.
Packaging Information
Xylistin available in vial of 10mL.
References
2. Ebbing Lautenbach and Ron E. Polk: Resistant gram-negative bacilli: A neglected healthcare crisis? Am J Health-Syst Pharm 2007; 64 (Suppl 14): S3-S21.
3. Gaynes R et al: Overview of nosocomial infections caused by gram negative bacilli. Clin Infect Dis 2005;41:848-854.
4. Adapted from Trends in Antimicrobial Resistance in Health Care Associated Pathogens and Effect on Treatment. L Clifford McDonald. Clin Infect Dis 2006; 42:S65-S71.
5. L.Silvia Munoz-Price and Robert A Weinstein: Current concepts acinetobacter infection. N Engl J Med 2008;358:1271-1281.
6. Ronald N Jones: Global epidemiology of antimicrobial resistance among community-acquired and nosocomial pathogens: A five-year summary from the SENTRY antimicrobial surveillance program (1997-2001). Semin RespirCrit Care Med 2003; 24(1):121-134.
7. Archibald LK. Gram-negative, hospital-acquired infections: a growing problem. Infect Control Hosp Epidemiol 2004; 25:809-811.
8. Ekta Gupta et al: Emerging resistance to carbapenems in a tertiary care hospital in north India. Indian J Med Res 2006; 124:95-98.
9. Taneja N, Maharwal S, Sharma M: Imipenem resistance in nonfermenters causing nosocomial urinary tract infections. Indian J Med Sci 2003; 57:294-299.
10. Navaneeth BV et al :A preliminary study on metallo-beta-lactamase producing Pseudomonas aeruginosa in hospitalized patients. Indian J Med Res 2002; 116:264- 7.
11. Mendiratta D.K, Deotale V and Narang P: Metallo-beta-lactamse producing Pseudomonas aeruginosa in hospital from rural area. Indian J Med Res 2005; 121:701-703.
12. Hemlatha V, Sekar U, Kamat V: Detection of metallo-betalactamase producing Pseudomonas aeruginosa in hospitalized patients. Indian J Med Res 2005; 122:148-52.
13. Gupta V, Datta P, ChanderJ: Prevalence of metallo-beta lactamase (MBL) producing Pseudomonas spp. andAcinetobacterspp. in a tertiary care hospital in India. J Infect 2006 May;52(5):311-4.
14. A Manoharan et al: Carbapenem resistant among Pseudomonas aeruginosa isolates in Indian medical centres: A preliminary report of Surveillance of Antimicrobial Resistance in India (SARI) study.
15. Mary V Jesudason et al: Comparison of two methods to detect carbapenemase and metal-beta-lactamase production in clinicalisolates. Indian J Med Res 2005; 121:780-783.
16. I Ratnam, C Franklin, D W Spelman: In vitro activities of 'new' and 'conventional' antibiotics against multi-drug resistant Gram negative bacteria from patients in the intensive care unit. Pathology 2007; 39 (6):586-8.
17. M Sinha, H Srinivasa: Mechanisms of resistance to carbapenems in meropenem resistant Acinetobacter isolates from clinical samples. Indian J Med Microbio 2007; 25(2):121-125.
18. P Gladstone et al: Incidence of carbapenem resistant nonfermenting gram negative bacilli from patients with respiratory infections in the intensive care units. Indian J Med Microbiol 2005; 23(3): 189-191.
19. Su Young Lee, Joseph L Kuti and David P Nicolau: Polymyxins : Older antibiotics for a new threat. Connecticut Medicine 2006; 70(1):25-28.
20. Robert EWHancock. Peptide antibiotics. The Lancet 1997; 349(9049):418-422.
21. Martin E Evans et al: Polymyxin B sulphate and colistin: Old antibiotics for emerging multiresistant gram negative bacteria. Ann Pharmacother 1999; 33:960-967.
22. Product Info Colomycin; Forest Laboratories UK Ltd May 2006.
23. Andrea Kwa et al: Polymyxin B: similarities to and differences from colistin (polymyxinE). Expert Rev Anti Infect Ther2007; 5(5): 811-821.
24. Gales et al: Contemporary assessment of antimicrobial susceptibility testing methods for polymyxin B and colistin: Review of available interpretative criteria and quality control guidelines. J Clin Microbio2001;39(1):183-191.
25. S.Chitnis et al: In vitro synergistic activity of colistin with aminoglycosides, beta-lactams and rifampin againstmultidrug-resistantgram-negative bacteria. J.Chemother2007; 19 (2): 226-229
26. Gunderson et al: Synergistic activity of colistin and ceftazidime against multiantibiotic-resistant Pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob Agents Chemother 2003; 47(3): 905-909.
27. Joon Young Song et al: In vitro activities of carbapenem/sulbactam combination, colistin, colistin/rifampicin combination and tigecycline against carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother 2007; 60 (2): 317-322
28. Product Info Colymycin; Parke Davis
29. Jimenez-Mejias ME et al: Cerebrospinal fluid penetration and pharmacokinetic/pharmacodynamic parameters of intravenously administered colistin in a case of multidrug-resistant Acinetobacter baumannii meningitis. Eur J Clin Microbiol Infect Dis 2002; 21 '.212-214.
30. Sohini Sarkaret al: Resurgence of colistin use. Am J Health-Syst Pharm 2007; 64:2462-2466.
31. Jian Li et al: Colistin: the re-emerging antibiotic for multidrug-resistant gram-negative bacterial infections. Lancet Infect Dis 2006; 6(9): 589-601.
32. Guidelines for the Management of Adults with Hospital-acquired, Ventilator-associated, and Healthcare-associated Pneumonia. Am J Respir Crit Care Med 2005; 171:388-416.
33. C Tascini et al: Clinical and microbiological efficacy of colistin therapy alone or in combination as treatment for multidrug resistant Pseudomonas aeruginosa diabetic foot infections with or without osteomyelitis. J Chemother 2006; 18(6):648-651.
34. Peter K. Linden, Shimon Kusne et al: Use of parenteral colistin for the treatment of serious infection due to antimicrobial-resistant Pseudomonas aeruginosa. Clin Infect Dis 2003; 37:e 154-160.
35. H Kallel et al: Colistin as a salvage therapy for nosocomial infections caused by multidrug-resistant bacteria in the ICU. International JAntimicrob Agents 2006; 28:366-369.
36. Koomanachai P, et al: Efficacy and safety of colistin (colistimethate sodium) for therapy of infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii in Siriraj Hospital Bangkok, Thailand. Int J infect Dis 2007; 11(5):402-406
37. J Garnacho et al: Treatment of multidrug-resistant Acinetobacter baumannii ventilator associated pneumonia (VAP) with intravenous colistin: A comparison with imipenem-susceptible VAP Clin Infect Dis 2003; 36:1111-1118.
38. Jeremy Goverman et al: Intravenous colistin for the treatment of multi-drug resistant, gram negative infection in the pediatric burn population. J Burn Care Res 2007; 28:421-426.
39. Argyris Michalopouios et al: Aerosolized colistin as adjunctive treatment of ventilator-associated pneumonia due to multidrug-resistant gram-negative bacteria: A prospective study. Resp Med 2008: 102:407-412.
40. KwaAL et al: Nebulized colistin in the treatment of pneumonia due to multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Clin Infect Dis 2005; 41:754-757.
41. Roger L Nation andJian Li: Optimizing use ofcolistin and polymyxin B in the critically ill. Semin Respir Crit Care Med 2007; 28:604-614.
42. Stein A, RaoultD: Colistin: an antimicrobial for the 21st century? Clin Infect Dis 2002; 35(7):901-902.
43. Matthew E Falagas et at Toxicity after prolonged (more than four weeks) administration of intravenous colistin. BMC Infect Dis 2005; 5:7.
44. Falagas ME, Kasiakou SK: Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Critical Care 2006; 10.R27.
45. Price DJE, Graham Dl: Effects of large doses ofcolistin sulphomethate sodium on renal function. Brit Med J1970; 4:525-527.
46. Li J et al: Pharmacokinetics of colistin methatesulfonate and colistin in a critically ill patient receiving continuous venovenous hemodiafiltration. Antimicrob Agents Chemother 2005; 49(11): 4814-4815.



 /a>
/a>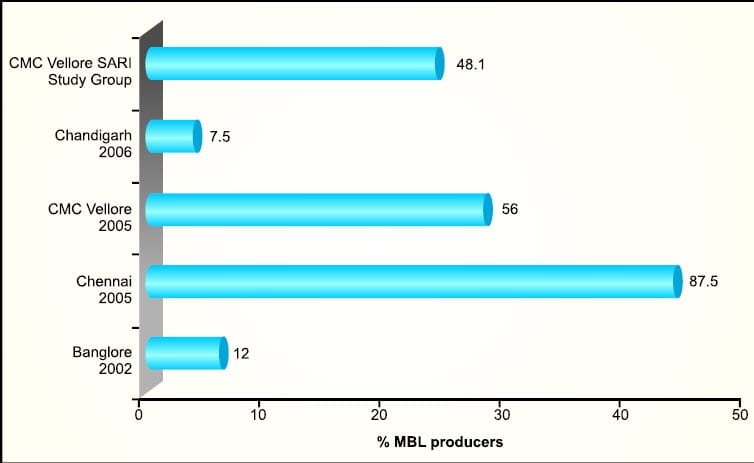
 /a>
/a>