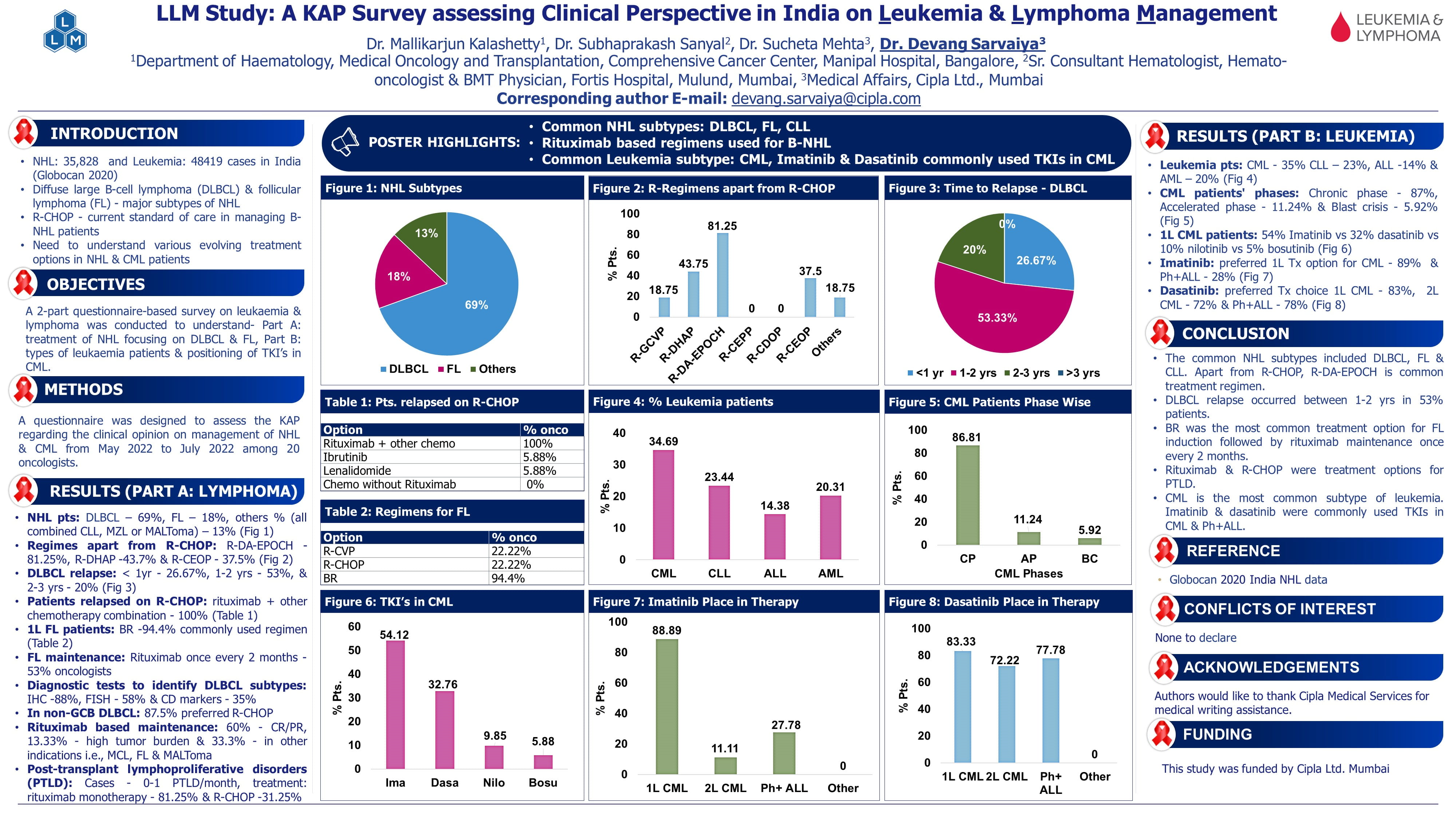
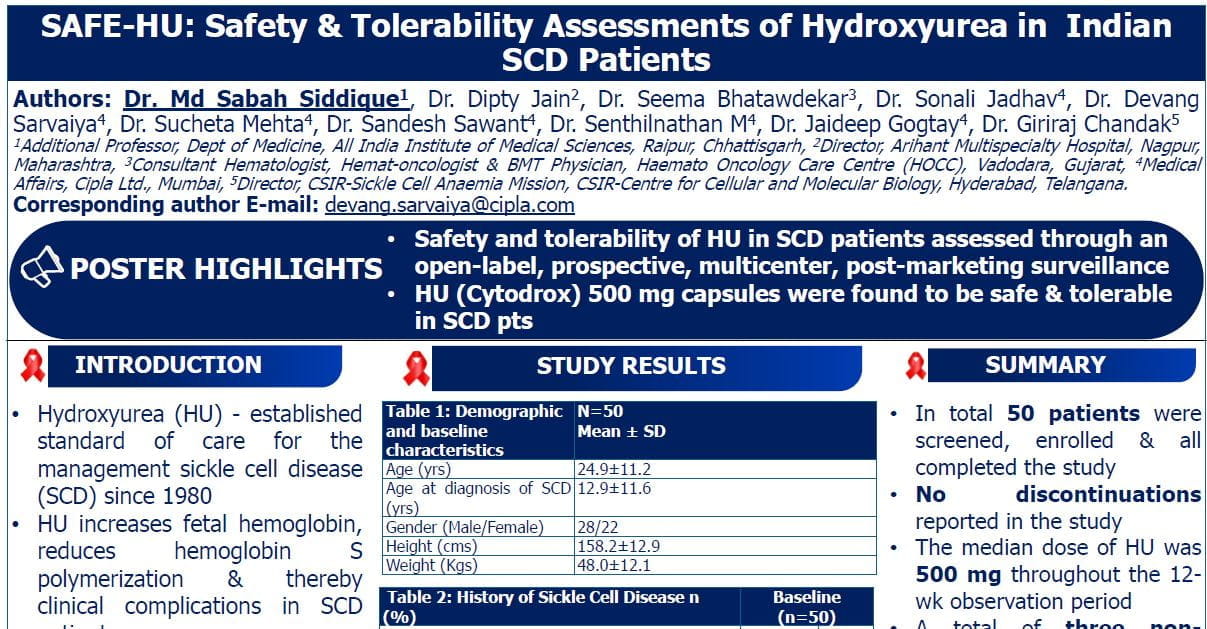
Key Abstracts in MDS & Leukemia
ASC4FIRST, A Pivotal Phase 3 Study of Asciminib (ASC) vs Investigator-Selected Tyrosine Kinase Inhibitors (IS TKIs) in Newly Diagnosed Patients (pts) with Chronic Myeloid Leukemia (CML): Primary Results- Hughes TP
Background
The study presents primary results from ASC4FIRST (NCT04971226), the first study in CML comparing all current standard-of-care frontline TKIs with a novel agent, asciminib (STAMP inhibitor), in newly diagnosed pts.
Methods
- Adults with CML were randomly assigned 1:1 to receive ASC 80 mg once daily or an IS TKI at standard label doses, stratified by ELTS risk category and pre-randomization selected (PRS) TKI (imatinib [IMA] or second-generation [2G] TKIs), which was selected by investigators before randomization, accounting for pt preference.
- Pts diagnosed within 3 mo before enrollment with no prior treatment (Tx) except IMA/2G TKIs for ≤2 wk prior to randomization were eligible.
- Primary objectives: To demonstrate superior major molecular response (MMR) rate at wk 48 with ASC vs IS TKI and ASC vs IS TKI within the stratum of pts with IMA as PRS TKI (ASCIMA vs IS TKIIMA).
- Secondary objective: Comparing MMR rate of ASC vs IS TKI at wk 48 within the stratum of pts with 2G TKIs as PRS TKI (ASC2G vs IS TKI2G).
Results
- Pts received ASC (n=201: ASCIMA, n=101; ASC2G, n=100) or IS TKI (n=204: IS TKIIMA, n=102; IS TKI2G, n=102 [nilotinib, 48%; dasatinib, 41%; bosutinib, 11%]).
- Median follow-up:16.3 and 15.7 mo for ASC and IS TKI, respectively (cutoff: Nov 28, 2023).
- At cutoff, Tx was ongoing in 86%, 62%, and 75% of pts on ASC, IMA, and 2G TKIs, respectively, with pts most commonly discontinuing due to unsatisfactory therapeutic effect (6%, 21%, 10%) (Tx failure per ELN2020 [5%, 16%, 8%], MMR loss [0.5%, 0%, 0%], physician decision [0.5%, 5%, 2%]) and adverse events (AEs) (5%, 11%, 10%). MMR rate at wk 48 (per ITT) was superior with ASC (67.7%) vs IS TKI (49.0%) and with ASCIMA (69.3%) vs IS TKIIMA (40.2%), meeting both primary objectives with high statistical significance; rate difference was 18.9% [95% CI, 9.6%-28.2%] and 29.6% [95% CI, 16.9%-42.2%], respectively, both with adjusted 1-sided P<.001.
- MMR rate at wk 48: Higher with ASC2G vs IS TKI2G (66.0% vs 57.8%). BCR::ABL1IS≤1% rate at wk 48 was 87% with ASC vs 73% with IS TKI and 84% with ASCIMA vs 62% with IS TKIIMA.
- At wk 48, MR4 and MR4.5 rates: Higher with ASC vs IS TKI (39% vs 21%; 17% vs 9%), ASCIMA vs IS TKIIMA (43% vs 15%; 18% vs 5%), and ASC2G vs IS TKI2G (35% vs 26%; 16% vs 13%).
- ASC had markedly favorable safety and tolerability vs IMA and 2G TKIs, with less grade ≥3 AEs (38%, 44%, 55%), half the rate of AEs leading to Tx discontinuation (5%, 11%, 10%), and less dose adjustments/interruptions to manage AEs (30%, 39%, 53%).
- The rate of arterial occlusive events was 1%, 0%, and 2%, respectively.
Conclusion
ASC showed a statistically significant superior efficacy and excellent safety and tolerability vs all current standard-of-care frontline Tx, with potential to be the therapy of choice for CML.
American Society of Clinical Oncology (ASCO) 2024 Congress. 31st May – 4th June 2024. Chicago, IL. Abstract No LBA6500
Long-term Efficacy of High-Dose Imatinib in Hispanic Patients without Access to Second-Generation Tyrosine Kinase Inhibitors Treated in LATAM centers- Trevino ED
Background
Treatment for patients with chronic myeloid leukemia (CML) had a benchmark with the introduction of imatinib, a tyrosine kinase inhibitor (TKI). Nevertheless, resistance due to genetic mutations in the BCR::ABL1 kinase receptor remains relatively common for many patients, leading to failure, lack of response, or suboptimal response. Ideally, to bypass genetic resistance, guidelines recommend switching to a second-generation TKI, but for many developing countries, socioeconomic barriers hinder the possibility of switching medication. Despite this scenario, scarce information is available to evaluate the clinical prognosis of these patients.
Methods
- A retrospective cohort analysis was conducted to compare the overall mortality of patients with CML who developed a suboptimal response to standard-dose imatinib and were treated with either high-dose imatinib or a second-generation TKI.
- A marginal structural model was created with inverse probability weighting and stabilized weights and depicted the survival curves and median using the Kaplan-Meier estimator.
- Primary outcome: Overall survival (OS) at 150 months, defined as the number of deaths once patients had reached treatment response based on parameters of the European Leukemia Net Guidelines for CML.
- Secondary outcomes: Disease-free survival (DFS) at 150 months, defined as the number of months after patients achieved either major molecular response or deep molecular response, and adverse events that included gastrointestinal, dermatologic, hematologic, and others.
Results
- The cohort included 148 patients, of which 32 received high-dose imatinib and 116 a second-generation TKI. While the study found no difference in the 150-month risk in both OS and DFS (Table), patients receiving second-generation TKI had an increase in median survival of OS (p-value = 0.009).
- No difference was found in the median survival of DFS (p-value = 0.55).
- No difference was found in either hematologic, gastrointestinal, dermatologic, or other adverse events (p-values of 0.39, 0.94, 0.24, and 0.33, respectively).
Conclusions
Ideally, patients who develop a suboptimal response to imatinib should be switched to a second-generation TKI. However, if impossible, study findings suggest that patients treated with high-dose imatinib have a similar OS and DFS prognosis to those receiving a second-generation TKI.
|
Estimates for primary and secondary outcomes. |
|||||
|
Type of Estimate |
Overall Survival |
Free-Disease Survival |
|||
|
Estimate |
95% CI |
Estimate |
95% CI |
||
|
Risk Ratio |
0.91 |
0.55 – 1.95 |
1.02 |
0.53 – 2.71 |
|
|
Risk Difference |
0.77 |
-0.3 – 0.21 |
0.01 |
-0.26 – 0.22 |
|
American Society of Clinical Oncology (ASCO) 2024 Congress. 31st May – 4th June 2024. Chicago, IL. Abstract No 6512
Updated Safety and Efficacy Data from the Phase 3 MANIFEST-2 Study of Pelabresib in Combination with Ruxolitinib for JAK Inhibitor Treatment-Naïve Patients with Myelofibrosis- Rampal R
Background
Pelabresib (PELA) is an oral, small-molecule, investigational BET inhibitor that aims to decrease expression of genes involved in MF. MANIFEST-2 (NCT04603495), a global, randomized, double-blind, Phase 3 study, investigated the efficacy and safety of PELA + ruxolitinib (PELA+RUX) vs placebo + RUX (PBO+RUX) in JAKi treatment-naïve patients (pts) with MF.
Methods
- Eligible pts had DIPSS score ≥ INT-1, platelet count ≥100 × 109/L, spleen volume ≥450 cm3, ≥2 symptoms with an average score ≥3 or total symptom score (TSS) ≥10 (MFSAF v4.0), peripheral blast count <5%, and ECOG PS ≤2.
- Pts were randomized 1:1. PELA or PBO was administered (QD for 14 consecutive days of 21) with RUX (BID for 21 days [1 cycle]).
- Primary endpoint: ≥35% spleen volume reduction from baseline (BL) (SVR35) at Week (Wk) 24.
- Secondary endpoints: Absolute change in TSS and ≥50% reduction in TSS from BL (TSS50) at Wk 24, and safety.
- Other endpoints: Hemoglobin (Hb) response (≥1.5 g/dL mean increase from BL without transfusions in the prior 12 wks), RBC transfusion number and bone marrow fibrosis (BMF).
Results
- N= 430 pts were randomized.
- At Wk 24, 65.9% (141/214) vs 35.2% (76/216) (p<0.001) of pts had an SVR35 response in the PELA+RUX vs PBO+RUX arms, respectively.
- SVR35 responders at any time: 80.4% (172/214) vs 50.0% (108/216); 80% (137/172) vs 63% (68/108) of responders reached SVR35 at Wk 12 scan; 83.7% (144/172) vs 79.6% (86/108) maintained response at cutoff.
- Mean change in absolute TSS: -15.99 (SE 1.028) vs -14.05 (SE 0.986) (p=0.0545), and TSS50: 52.3% (112/214) vs 46.3% (100/216) (p=0.216) at Wk 24.
- 2-fold difference in pts with both SVR35 and TSS50 with PELA+RUX (40.2% [86/214]) vs PBO+RUX (18.5% [40/216]). Hb response occurred in 10.7% (23/214) vs 6.0% (13/216) of pts, with differences in mean Hb levels maintained at 48 wks.
- In pts with anemia (Hb BL <10 g/dL), Hb response occurred in 16.4% (11/67) vs 14.1% (10/71). A total of 30.8% (66/214) vs 39.8% (86/216) of required RBC transfusion during the first 24 wks. BMF improvement ≥1 grade occurred in 38.5% (40/104) vs 24.2% (24/99) of pts (odds ratio 2.09; p=0.019).
- Of 426 pts evaluated for safety, the most common treatment-emergent AEs (≥20%) in the PELA+RUX vs PBO+RUX arms were anemia (43.9% vs 55.6% [Grade ≥3, 23.1% vs 36.4%]), thrombocytopenia (32.1% vs 23.4% [9% vs 5.6%]), platelet count decreased (20.8% vs 15.9% [4.2% vs 0.9%]), and diarrhea (23.1% vs 18.7% [0.5% vs 1.4%]).
Conclusions
PELA+RUX significantly and durably reduced splenomegaly, with a trend toward reduced TSS, and improved anemia and BMF at Wk 24 compared with PBO+RUX in JAKi treatment-naïve pts with MF, addressing key hallmarks of MF. Results support a potential paradigm shift to combination therapy for MF.
American Society of Clinical Oncology (ASCO) 2024 Congress. 31st May – 4th June 2024. Chicago, IL. Abstract No 6502
Obecabtagene autoleucel (obe-cel, AUTO1) in Adults with Relapsed/Refractory B-Cell Acute Lymphoblastic Leukemia (R/R B-ALL): Overall Survival (OS), Event-Free Survival (EFS) and the Potential Impact of Chimeric Antigen Receptor (CAR)-T cell Persistency and Consolidative Stem Cell Transplantation (Sct) in the Open-Label, Single-Arm FELIX Phase IB/II Study- Jabbour E
Background
Obe-cel is an autologous CAR-T cell product with a fast off-rate CD19 binder designed to mitigate immunotoxicity and improve expansion/persistence. The study reported OS and EFS in all patients (pts) treated with obe-cel, alongside the impact of CAR-T cell persistency and consolidative SCT for pts in remission.
Methods
Pts aged ≥18 yrs with R/R B-ALL were enrolled. CAR-T products were generated via an automated process (Roddie C et al. Blood 2023;142[Suppl 1]:222). Pts received bridging therapy as appropriate and underwent lymphodepletion (fludarabine, 4×30mg/m2; cyclophosphamide, 2×500mg/m2), followed by obe-cel split dose infusions on Days 1 and 10 based on pre-lymphodepletion leukemic burden at a target dose of 410×106 CAR-T cells.
Results
- A total of 127/153 (83%) enrolled pts were infused.
- At screening, pts’ median age was 47 yrs; 42%/31%/44% had received prior blinatumomab/inotuzumab ozogamicin/allogeneic SCT; median bone marrow blast burden was 36% (range: 0−100).
- At data cut-off (13 September 2023), median follow-up was 16.6 mos (range 3.7−36.6 mos).
- The overall complete remission or complete remission with incomplete count recovery rate among infused pts was 78%.
- Among responding pts, 17/99 (17%) proceeded to consolidative SCT while in remission; all 17 (100%) were in measurable residual disease (MRD)-negative remission (≤10–4 leukemic blasts) and 10/17 (59%) showed CAR-T cell persistency prior to SCT.
- Loss of CAR-T cell persistency was associated with a hazard risk of relapse or death 2.9 times compared with pts who had ongoing CAR-T cell persistency.
- Pts who experienced B-cell recovery had a hazard risk of relapse or death 1.7 times compared with pts without B-cell recovery.
- At 12 mos, the EFS rate was 50% and 43% with or without censoring for consolidative SCT or new therapies, respectively; the OS rate was 61% and 59% with or without censoring for SCT, respectively.
Conclusions
Ongoing CAR-T cell persistency and B-cell aplasia were associated with improved EFS without further consolidation post-obe-cel. At the current follow-up, consolidative SCT for pts in MRD-negative remission post-obe-cel did not improve EFS or OS.
American Society of Clinical Oncology (ASCO) 2024 Congress. 31st May – 4th June 2024. Chicago, IL. Abstract No 6504
Key Abstracts in Myeloma
Phase 3 Randomized Study of Isatuximab (Isa) Plus Lenalidomide and Dexamethasone (Rd) with Bortezomib Versus Isard in Patients with Newly Diagnosed Transplant Ineligible Multiple Myeloma (NDMM TI)- Leleu XP
Background
CD38 targeting immunotherapy is approved in combination with lenalidomide and dexamethasone in NDMM TI and considered the current standard of care (SOC). The best treatment combinations are important in NDMM TI, as outcomes worsen with successive lines of therapy. To improve current SOC, the study evaluated the added value of prolonged use of bortezomib for 18 months with reduced intensity weekly schedule to IsaRd, with the intent to demonstrate the impact of a PI in a quadruplet regimen to improve depth of response. In BENEFIT/IFM2020-05 study (NCT04751877), the study investigated efficacy and safety of IsaRd vs Isa-VRd in NDMM TI.
Methods
- BENEFIT is a prospective, multicenter, randomized, parallel trial.
- Patients aged 65-79, non-frail, with NDMM TI were randomized 1:1 and stratified by age, high-risk cytogenetic and center.
- Isa-VRd arm received V (1.3 mg/m2 SC weekly up to c12 (c), bimonthly up to c18); both arms received Isa (10 mg/kg IV weekly and bimonthly up to c12, then monthly), R (25 mg), and d (20 mg up to c12).
- Primary endpoint: Minimal residual disease (MRD) 10-5 negative rate (NGS) at 18 months from treatment start analyzed in ITT.
- Secondary endpoints: Survival times (OS, PFS, EFS, TTNT), response rates and durations, MRD endpoints, and safety (using NCI CTCAE v5.0).
Results
- At data cutoff date (02 Feb 2024), 270 patients (135 per arm) were recruited.
- Patients baseline characteristics were well balanced across arms, overall median age was 73.2 years [IQR. 71;76], 90 patients (33%) were >75 years, 23 (9%) had high-risk cytogenetic (IFM score >1), 181 (76%) had R-ISS2+3, and 47 (17%) had impaired renal function (eGFR <60 mL/min).
- MRD negativity rates at 10-5 at 18 months were significantly higher in Isa-VRd arm compared to IsaRd arm (47% vs 24%, OR for negative MRD =2.96 [95%CI. 1.73 – 5.07, p<0.001].
- The MRD benefit was consistent across subgroups. At 21.2 months median follow-up, 33 (12%) patients had relapsed and 20 (7%) had died, and no significant difference were observed across arms, yet.
- The addition of weekly “light” schedule of bortezomib did not significantly affect relative dose intensity of IsaRd.
- Forty-four (33%) patients presented with neurological adverse events grade ≥2 in the Isa-VRd vs 27 (20%) in IsaRd arm.
Conclusions
Isa-VRd significantly deepened responses including a significant increase of the MRD negative rate at 10-5 vs IsaRd. The safety profile is consistent with addition of bortezomib. This study supports Isa-VRd as a new standard of care for NDMM TI non-frail patients.
|
N (%) (ITT Population) |
IsaRd(n=135) |
Isa-VRd (n=135) |
p-value |
|
≥CR |
24 (18) [12 – 25] |
54 (40) [32 – 49] |
0.0001 |
|
≥CR- MRD- 10-5 |
16 (12) [7 – 19] |
29 (21) [15 – 29] |
0.04 |
|
MRD- 10-6 |
20 (15) [9 – 22] |
46 (34) [26 – 43] |
0.0004 |
|
PFS at 18 months |
86% [80 – 92] |
87.2% [82 – 93] |
0.47 |
|
OS at 18 months |
93.6% [90 – 98] |
92.4% [88 – 97] |
0.77 |
American Society of Clinical Oncology (ASCO) 2024 Congress. 31st May – 4th June 2024. Chicago, IL. Abstract No 7501
Phase 3 Study Results of Isatuximab, Bortezomib, Lenalidomide, and Dexamethasone (Isa-VRd) versus VRd for Transplant-Ineligible Patients with Newly Diagnosed Multiple Myeloma (IMROZ)- Facon T
Background
The first line of treatment (tx) is important for patients (pts) with newly diagnosed multiple myeloma (NDMM) as pts may not have a chance for subsequent therapy.
VRd is currently a standard of care (SOC) in NDMM. Isa is an approved anti-CD38 monoclonal antibody (mAb) inducing myeloma cell death through multiple mechanisms. The Phase 3 IMROZ study (NCT03319667), investigated efficacy and safety of Isa-VRd vs VRd in transplant-ineligible NDMM pts.
Methods
- IMROZ is a global, prospective, randomized, open-label study done at 102 study sites in 21 countries.
- Included pts had active, measurable NDMM not considered for transplant due to elderly age or comorbidities.
- Pts aged ≥80 were excluded.
- Pts were randomized 3:2 and stratified by age, R-ISS stage and China vs non-China, to receive Isa-VRd or VRd. Isa-VRd arm pts received Isa (10 mg/kg IV); both arms received V (1.3 mg/m2 SC), R (25 mg PO) and d (20 mg IV/PO).
- Primary endpoint: Progression-free survival (PFS).
- Key secondary endpoints: Complete response (CR), minimal residual disease negativity (MRD-) (10-5 by NGS) in pts with CR, very good partial response or better and overall survival.
- Adverse events (AEs) and laboratory parameters were graded with NCI CTCAE v4.03.
Results
- 446 pts (265 Isa-VRd, 181 VRd) were randomized; pt characteristics were well balanced.
- At data cutoff (26 Sep 2023), 125 (47.2%) and 44 (24.3%) pts in Isa-VRd and VRd arms were still on tx, respectively.
- Median (mdn) tx duration was 53.2 (Isa-VRd) vs 31.3 (VRd) mo; addition of Isa did not significantly affect relative dose intensity of VRd.
- At mdn follow-up of 59.7 mo, mdn PFS was not reached (Isa-VRd) vs 54.3 mo (VRd); HR 0.596 (98.5% CI 0.406–0.876), log-rank p=0.0005.
- From the current trend, projected Isa-VRd mdn PFS will reach ~90 mo. PFS benefit was consistent across subgroups and maintained through subsequent line of therapy (PFS2 HR 0.697; 95% CI: 0.51-0.952).
- Isa-VRd led to deep and sustained responses and was well-tolerated. Exposure-adjusted Grade 5 TEAE rate was 0.03 (Isa-VRd) vs 0.02 (VRd).
Conclusions
IMROZ is the first Phase 3 study of an anti-CD38 mAb with SOC VRd in this pt population to show a significantly reduced risk of progression or death by 40.4% vs VRd while providing deep and sustained responses. The safety profile was consistent with addition of Isa to VRd. Numerical differences in TEAEs are largely explained by longer exposure in the Isa-VRd arm. These results support Isa-VRd as a potential new SOC in pts not intended for transplant.
|
% pts |
Isa-VRd (n=265) |
VRd (n=181) |
Stratified Odds Ratio (95% CI) |
1-Sided |
|
CR |
74.7 |
64.1 |
1.656 |
0.008 |
|
MRD- CR |
55.5 |
40.9 |
1.803 |
0.0013 |
|
Sustained MRD- for at least 12 mo |
46.8 |
24.3 |
2.729 |
<0.0001 |
|
Grade ≥3 TEAE |
91.6 |
84.0 |
- |
|
|
Grade 5 TEAE |
11.0 |
5.5 |
||
|
Any TEAE leading to definitive tx discontinuation |
22.8 |
26.0 |
American Society of Clinical Oncology (ASCO) 2024 Congress. 31st May – 4th June 2024. Chicago, IL. Abstract No 7500
Daratumumab (DARA) + Bortezomib/Lenalidomide/Dexamethasone (VRd) in Transplant-Eligible (TE) Patients (pts) with Newly Diagnosed Multiple Myeloma (NDMM): Analysis of Minimal Residual Disease (MRD) in the PERSEUS Trial- Otero PR
Background
In the primary analysis of the phase 3 PERSEUS study, subcutaneous DARA (DARA SC) + VRd (D-VRd) induction/consolidation (ind/consol) and D-R maintenance improved progression-free survival (PFS) and increased depth of response (complete response or better [≥CR] and MRD negativity [neg]) compared to VRd ind/consol and R maintenance for TE NDMM. The study reported further results on deepening of response and MRD neg during maintenance.
Methods
- TE pts with NDMM were randomized 1:1 to D-VRd or VRd. Pts in both arms received up to six 28-day cycles (4 pre-ASCT ind, 2 post-ASCT consol) of VRd (V 1.3 mg/m2 SC on Days [D] 1, 4, 8, 11; R 25 mg PO on D 1-21; d 40 mg PO/IV on D 1-4, 9-12) followed by R maintenance (10 mg PO on D 1-28 until progressive disease [PD]).
- Pts in the D-VRd arm also received DARA SC (DARA 1,800 mg + recombinant human hyaluronidase PH20 [rHuPH20; 2,000 U/mL; Halozyme]) QW in Cycles 1-2, Q2W in Cycles 3-6, and Q4W during maintenance until PD.
- MRD-neg rate (clonoSEQ) was defined as the proportion of ITT pts who achieved both ≥CR and MRD neg.
Results
- In the 709 pts randomized (D-VRd, n=355; VRd, n=354), responses deepened over time with D-VRd vs VRd, including rates of ≥CR (end of consol: 44.5% vs 34.7%; P= 0.0078 and overall: 87.9% vs 70.1%; P<0.0001).
- MRD-neg rates increased over time and were higher with D-VRd vs VRd at 12, 24, and 36 mo after Cycle 1 Day 1 (all P<0.0001; Table). Rates of sustained MRD neg for ≥12 mo were higher for D-VRd vs VRd (10–5: 64.8% vs 29.7%; P<0.0001; 10–6: 47.3% vs 18.6%; P<0.0001); results were consistent across prespecified clinically relevant subgroups.
- Among pts who were MRD positive (pos) at end of consol, significantly higher proportions of pts in the D-VRd group vs the VRd group achieved MRD neg during maintenance at 10–5 (68.8% vs 52.7%; P= 0.0330) and 10–6 (62.3% vs 31.0%; P<0.0001) and sustained MRD neg for ≥12 mo at 10–5 (44.2% vs 22.6%; P= 0.0028) and 10–6 (34.4% vs 12.7%; P<0.0001).
- End of consol and overall MRD neg at both 10–5 and 10–6 were associated with improved PFS.
Conclusions
During maintenance, a greater proportion of pts with MRD-pos status achieved MRD neg with D-R vs R. The higher rates of deep (10–6) and sustained MRD neg achieved with D-VRd ind/consol and D-R maintenance vs VRd ind/consol and R maintenance translated to a clinically meaningful benefit of improved PFS. These data further support D-VRd and D-R maintenance as a new standard of care for TE pts with NDMM and highlight the benefit of DARA SC in maintenance.
|
10–5 |
10–6 |
|||||
|
D-VRd |
VRd |
P |
D-VRd |
VRd |
P |
|
|
Rates of MRD neg up to: |
||||||
|
12 mo |
65.1% |
38.7% |
<0.0001 |
43.9% |
20.9% |
<0.0001 |
|
24 mo |
72.1% |
44.9% |
<0.0001 |
57.7% |
27.4% |
<0.0001 |
|
36 mo |
74.6% |
46.9% |
<0.0001 |
63.9% |
30.8% |
<0.0001 |
American Society of Clinical Oncology (ASCO) 2024 Congress. 31st May – 4th June 2024. Chicago, IL. Abstract No 7502
Results from the Randomized Phase 3 DREAMM-8 Study of Belantamab Mafodotin Plus Pomalidomide and Dexamethasone (BPd) vs Pomalidomide Plus Bortezomib and Dexamethasone (PVd) in Relapsed/Refractory Multiple Myeloma (RRMM)- Trudel S
Background
Use of triplet/quadruplet therapies for 1L MM raises the need for novel combinations at first relapse, which belantamab mafodotin (belamaf) combos may address. In DREAMM-7, BVd led to a significant improvement in progression-free survival (PFS) and a strong trend in improved overall survival (OS) vs daratumumab-Vd in patients (pts) with ≥1 prior therapy. The study reported results from DREAMM-8 (NCT04484623), which tested a different belamaf combo (BPd) and met its primary endpoint of independent review committee–assessed PFS at a prespecified interim analysis.
Methods
- DREAMM-8 is a phase 3, open-label, randomized, multicenter trial evaluating the efficacy and safety of BPd vs PVd in RRMM pts who received ≥1 prior line of therapy (LoT), including lenalidomide.
- Pts were randomly assigned 1:1 to BPd (28-d cycles): belamaf 2.5 mg/kg IV (D1, C1), 1.9 mg/kg (D1, C2+) + pom 4 mg (D1-21, all C) + dex 40 mg (D1, QW, all C), or PVd (21-d cycles): pom 4 mg (D1-14, all C) + bortezomib 1.3 mg/m2 SC (D1, 4, 8, 11 [C1-8]; and D1, 8 [C9+]) + dex 20 mg (day of and 1 day after bortezomib dose).
Results
- 155 pts were randomly assigned to BPd and 147 to PVd.
- With a median (range) follow-up of 21.78 mo (0.03-39.23), median PFS (95% CI) was not reached (NR; 20.6-NR) with BPd vs 12.7 mo (9.1-18.5) with PVd (HR, 0.52; 95% CI, 0.37-0.73; P<0.001).
- 12-month PFS rate (95% CI) was 71% (63-78%) with BPd vs 51% (42-60%) with PVd. ORR (95% CI) was 77% (70.0-83.7%) with BPd vs 72% (64.1-79.2%) with PVd; rate of complete response or better (95% CI) was 40% (32.2-48.2%) with BPd vs 16% (10.7-23.3%) with PVd. Median duration of response (95% CI) was NR (24.9-NR) with BPd vs 17.5 mo (12.1-26.4) with PVd.
- A positive trend favoring BPd was seen for OS (HR, 0.77; 95% CI, 0.53-1.14); follow up for OS is ongoing. Adverse events (AEs) were reported in >99% and 96% of pts in the BPd and PVd arms, respectively. Of pts treated with BPd, 89% had ocular AEs (CTCAE grade 3/4, 43%) vs 30% (grade 3/4, 2%) in the PVd arm.
- AEs were generally manageable, and broadly consistent with known safety profile of individual agents.
Conclusions
The DREAMM-8 study demonstrated a statistically significant and clinically meaningful PFS benefit with BPd vs PVd in RRMM with >1 prior LoT. BPd also led to deeper and more durable responses, showed a favorable OS trend, and had a manageable safety profile. Clinical trial information: NCT04484623.
|
Additional baseline and safety data. |
||
|
Baseline Characteristics |
BPd |
PVd |
|
Prior LoT, median (range) |
1 (1-6) |
1 (1-9) |
|
Prior antimyeloma therapy, n (%) |
||
|
Immunomodulator |
155 (100) |
147 (100) |
|
Proteasome inhibitor |
140 (90) |
136 (93) |
|
Anti-CD38 antibody |
38 (25) |
42 (29) |
|
Safety |
(n=150)a |
(n=145)a |
|
Grade 3/4 AEs, n (%) |
136 (91) |
106 (73) |
|
Any SAEs; fatal SAEs, n (%) |
95 (63); 17 (11) |
65 (45); 16 (11) |
|
AEs leading to discontinuation of any study treatment, n (%) |
22 (15) |
18 (12) |
aSafety data were evaluated in the safety analysis set.
American Society of Clinical Oncology (ASCO) 2024 Congress. 31st May – 4th June 2024. Chicago, IL. Abstract No LBA105
Key Abstracts in Lymphomas
Tucidinostat plus R-CHOP in Previously Untreated Diffuse Large B-Cell Lymphoma with Double Expression of MYC and BCL2: An Interim Analysis From the Phase III DEB Study- Zhao W
Background
Epigenetic dysregulation is commonly correlated with the pathogenesis and development in diffuse large B-cell lymphoma (DLBCL). Tucidinostat, a subtype-selective histone deacetylase (HDAC) inhibitor, has shown promising efficacy in combination with R-CHOP in DLBCL patients with double expression of MYC and BCL2 (DE) in exploratory studies.
Methods
- A randomized, double-blind, placebo-controlled, phase III trial (DEB) was conducted to evaluate the efficacy and safety of tucidinostat plus R-CHOP in comparison with R-CHOP in previously untreated DLBCL patients with DE.
- Patients were randomly assigned in a 1:1 ratio to receive six cycles of either tucidinostat plus R-CHOP (tucidinostat group) or placebo plus R-CHOP (placebo group).
- Patients who achieved complete response (CR) after combination therapy received either tucidinostat or placebo as maintenance treatment with a maximum duration of 24 weeks.
- Primary endpoint: Investigator-assessed event-free survival (EFS)
- Secondary endpoint: Complete response rate (CRR) evaluated at the end of combination treatment.
- An interim analysis was pre-defined to be conducted when CRR was obtained and the number of EFS events reached at least 60% of the total events required for entire study.
Results
- Between May 21, 2020, and July 25, 2022, 423 patients were enrolled and randomly assigned, 211 to the tucidinostat group and 212 to the placebo group. At data cutoff of this interim analysis (January 10, 2023), the median follow-up time was 13.9 months (95% CI, 12.9 - 15.4).
- A total of 152 EFS events (68.5% of the planned total) were observed, with 64 (30.3%) in the tucidinostat group and 88 (41.5%) in the placebo group. The 24-month EFS rate was 58.9% (95% CI, 48.9-67.6) in the tucidinostat group and 46.2% (95% CI, 35.7-56.1) in the placebo group.
- The hazard ratio (HR) between the two groups was 0.68 (95% CI, 0.49-0.94), with a p-value of 0.018. At the completion of combination treatment, the CRR in the tucidinostat and placebo groups were 73.0% (95% CI, 66.6-78.5) and 61.8% (95% CI, 55.1-68.1), respectively. The adjusted difference in CRR was 11.1% (95% CI, 2.3-20.0; P=0.014).
- The safety profiles of both groups were as expected, with no new safety findings.
- The incidence of ≥ grade 3 hematologic adverse events was generally higher in the tucidinostat group than the placebo group, but most patients were able to tolerate and complete the planned treatment cycles.
- No significant cardiac toxicity, hepatotoxicity, or nephrotoxicity were observed in both groups.
Conclusion
The DEB study is the first phase III trial to show that combining tucidinostat with R-CHOP regimen is a feasible and efficacious novel approach in previously untreated DLBCL patients with DE. Tucidinostat plus R-CHOP could be a new frontline treatment option in this patient population.
American Society of Clinical Oncology (ASCO) 2024 Congress. 31st May – 4th June 2024. Chicago, IL. Abstract No LBA7003
Brentuximab Vedotin In Combination With Lenalidomide And Rituximab In Patients With Relapsed/Refractory Diffuse Large B-cell Lymphoma: Results from the Phase 3 ECHELON-3 Study- Kim JA
Background
Despite recent advances, there remains a need for novel therapies for pts with R/R DLBCL. BV, an anti-CD30 antibody-drug conjugate, has shown efficacy and safety when combined with lenalidomide (len) and with rituximab (R) in heavily pretreated populations (Bartlett 2022; Ward 2022). The double-blind, global phase 3 ECHELON-3 study (NCT04404283) compared BV with R+len (R2) vs R2 in pts with R/R DLBCL who are ineligible for HSCT or CAR T-cell therapy. The study reported results from the interim analysis (IA) for overall survival (OS).
Methods
- Pts with R/R DLBCL received BV+R2 or placebo+R2 (randomized 1:1). Pts received BV (1.2 mg/kg) or placebo q3w, R (375 mg/m2) q3w, and len (20 mg) qd.
- Primary endpoint: OS in the intent-to-treat population.
- Secondary endpoints: Investigator-assessed progression-free survival (PFS), objective response rate (ORR), and complete response (CR) rate.
- The preplanned IA was performed at 134 OS events with a prespecified efficacy boundary of 2-sided P=0.0232.
Results
- 230 pts were randomized: 112 to BV+R2 and 118 to R2; all but 2 pts (both in R2 arm) received ≥1 dose of study drug.
- Median age was 71 yrs (range, 21-89), 56.5% were male, and 10.9% had an ECOG of 2. Median prior lines of therapy was 3 (range, 2-8); 29% had prior CAR T-cell therapy and 68% were CD30- (<1% CD30 tumor expression).
- At median follow-up of 16.4 months (mos) (range, 0.1-31.5) (cut-off: January 22, 2024), median OS was 13.8 mos (95% CI: 10.3-18.8) with BV+R2 vs 8.5 mos (95% CI: 5.4-11.7) with R2 (HR 0.629; 95% CI: 0.445-0.891; P=0.0085); OS benefit was consistent across key subgroups. Median PFS was 4.2 mos (95% CI: 2.9-7.1) with BV+R2 vs 2.6 mos (95% CI: 1.4-3.1) with R2 (HR 0.527; 95% CI: 0.380-0.729; P<0.0001). ORR was 64.3% (95% CI: 54.7-73.1) with BV+R2 vs 41.5% with R2 (95% CI: 32.5-51.0; P=0.0006); CR rate was 40.2% vs 18.6%, respectively.
- In CD30+ vs CD30- subgroups, ORR/CR was 72.2%/38.9% vs 60.5%/40.8% with BV+R2, respectively, and 50.0%/26.3% vs 37.5%/15.0% with R2, respectively. Efficacy analysis including cell of origin will be presented.
- The safety profile of BV+R2 was tolerable vs R2: Grade (Gr) ≥3 treatment-emergent adverse events (TEAEs) were 88% vs 77%, serious TEAEs were 60% vs 50%, and Gr 5 TEAEs were 12% vs 8%, respectively.
- Most common TEAEs were neutropenia (46% vs 32%), anemia (29% vs 27%), and diarrhea (31% vs 23%). Rates of peripheral neuropathy for BV+R2 vs R2 were 31% vs 24% (all Gr) and 6% vs 2% (Gr 3). Median treatment duration was 3.6 mos with BV+R2 vs 2.0 mos with R2.
Conclusions
Treatment with BV+R2 triplet, compared to R2, demonstrated statistically significant and clinically meaningful improvements in all key efficacy outcomes including OS in high-risk subgroups, with manageable safety. This triplet regimen represents a novel treatment option for pts with heavily pretreated R/R DLBCL.
American Society of Clinical Oncology (ASCO) 2024 Congress. 31st May – 4th June 2024. Chicago, IL. Abstract No LBA7005
Benefit of Rituximab Maintenance After First-Line Bendamustine-Rituximab In Mantle Cell Lymphoma- Wang Y
Background
Rituximab maintenance (RM) after first-line (1L) bendamustine and rituximab (BR) in MCL did not improve progression-free survival in the MAINTAIN trial (Rummel et al, ASCO 2016) but was associated with improved survival outcomes in a North American observational study (Martin et al, JCO 2023). The study was conducted to examine the potential benefit of RM after BR using a large observational cohort from 26 US academic centers.
Methods
- Patients with MCL who received 1L BR (without stem cell transplant) outside of clinical trials were included.
- At the landmark of 3 months after the end of BR, patients who achieved a complete response (CR) or partial response (PR) to BR and had no evidence of progressive disease (PD) or second-line (2L) therapy were deemed eligible for RM.
- Event-free survival (EFS) was defined as time from landmark to progression, relapse, retreatment, or death. EFS2 was defined as time from landmark to progression, relapse, or retreatment following 2L therapy or death.
- RM was not considered a line of therapy for either endpoint. OS was defined as time from landmark to death.
- Survival analysis was done with Kaplan-Meier methods and Cox regression models adjusting for sex and simplified MIPI.
Results
- Among 796 patients who received 1L BR in 2007-2020, 693 achieved a CR or PR. At the 3-month post-BR landmark, 613 had no evidence of PD, among whom 318 (52%) received RM and 295 did not.
- The RM group was younger (median age 70 vs 72, p = 0.010) and more predominantly male (78% vs 69%, p = 0.047). There was no statistical difference in stage, simplified MIPI, histology (blastoid or pleomorphic vs classic), Ki-67, TP53 alteration, complex karyotype, year of BR start, or best response to BR between the two groups.
- The median follow-up after the 3-month post-BR landmark was 61.3 months (95% CI 62.6-70.4). The median number of doses of RM was 10 (IQR 5-12). RM was associated with improved EFS (median 47.1 vs 29.7 months, adjusted HR 0.59, 95% CI 0.48-0.73), EFS2 (median 89.1 vs 48.3 months, adjusted HR 0.63, 95% CI 0.50-0.81), and OS (median 136.1 vs 74.3 months, adjusted HR 0.57, 95% CI 0.44-0.75) (all p values < 0.001). In patients with CR to 1L BR (n=527), 271 (51%) received RM, for a median of 11 (IQR 6-12) doses.
- In this subgroup, RM was associated with improved EFS (median 60.6 vs 31.5 months, adjusted HR 0.56, 95% CI 0.44-0.71), EFS2 (median 89.1 vs 48.3 months, adjusted HR 0.62, 95% CI 0.48-0.81), and OS (median 136.1 vs 75.6 months, adjusted HR 0.59, 95% CI 0.44-0.79) (all p values < 0.001).
- Analysis in patients with PR to 1L BR was limited by the sample size (n=86, 47 received RM).
- The numeric differences in median EFS (20.8 vs 11.5, log-rank p = 0.370), EFS2 (48.9 vs 30.3, log-rank p = 0.210) and OS (87.3 vs 46.9, log-rank p = 0.067) were not statistically significant.
Conclusions
In this large multicenter study, RM after 1L BR was associated with improved EFS and OS, supporting its use in routine practice for patients with newly diagnosed MCL.
American Society of Clinical Oncology (ASCO) 2024 Congress. 31st May – 4th June 2024. Chicago, IL. Abstract No 7006