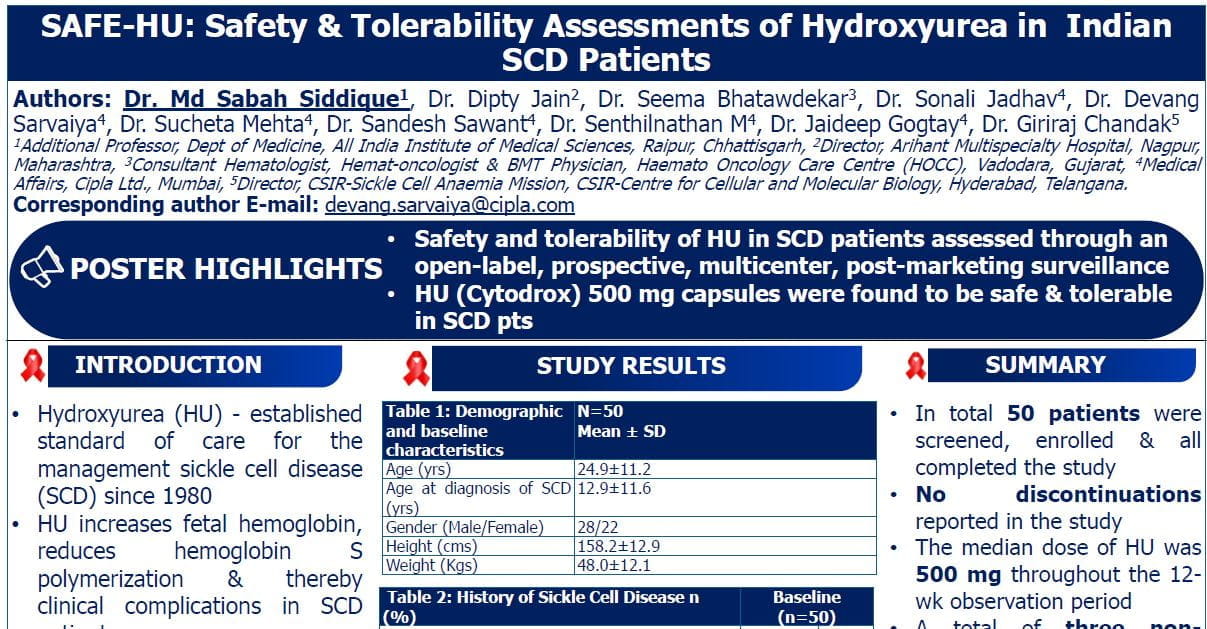
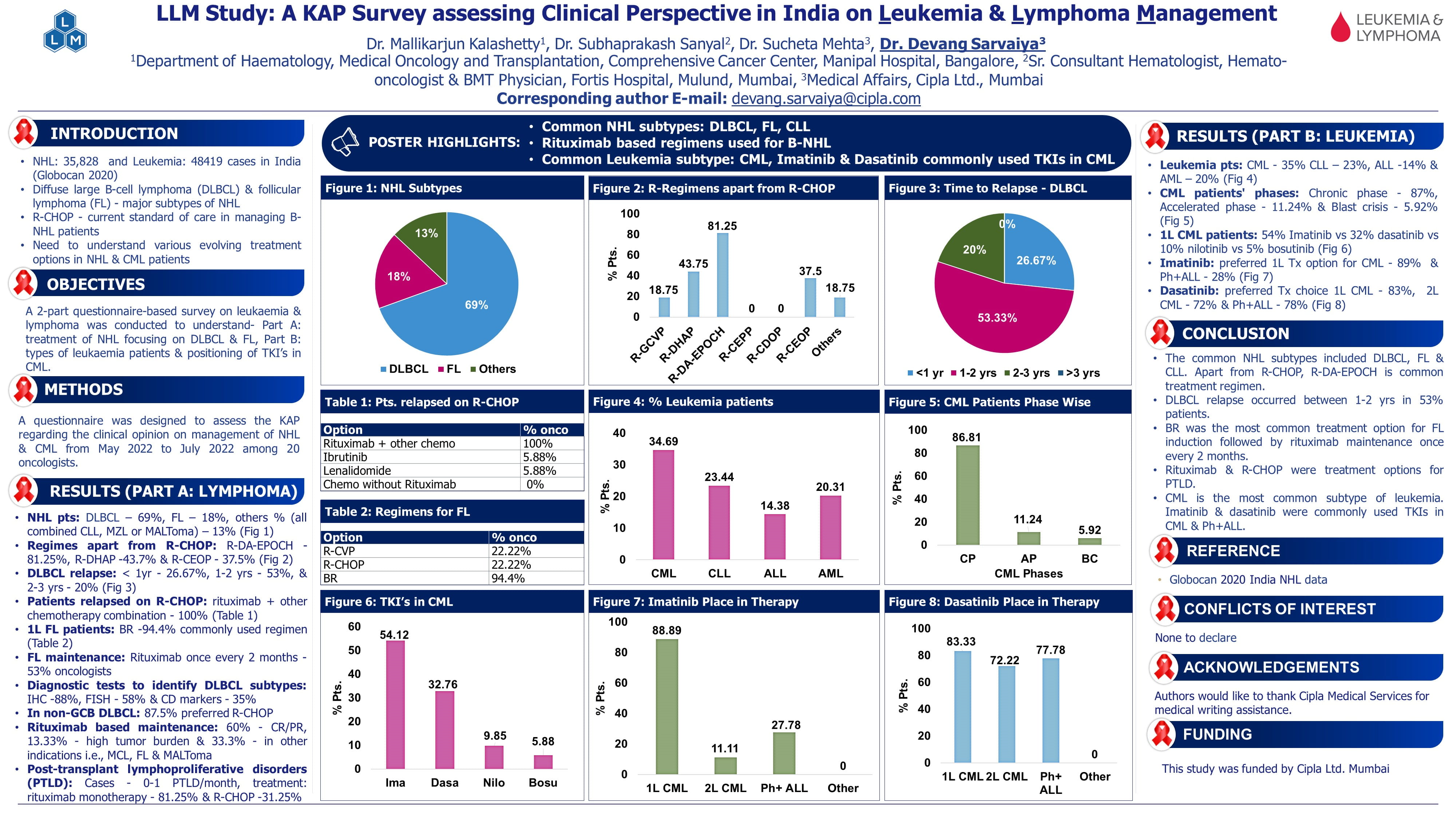
A Phase II Randomized Trial Comparing Low-Dose Cytarabine and Venetoclax +/- Midostaurin in Non-Adverse Cytogenetic Risk Acute Myeloid Leukemia: The ALLG AMLM25 Intervene Trial
Speaker: Chong Chua, Monash University, Australia
Key Highlights:
Introduction:
In older patients with newly diagnosed acute myeloid leukemia (AML), FLT3 mutations are identified in approximately 15-20% of cases. For patients receiving venetoclax-based therapies such as AZA-VEN, FLT3-ITD is associated with intermediate clinical benefit, as per the latest ELN classification. However, FLT3-ITD mutations are a known mechanism of resistance following venetoclax therapy, with about 20% of patients exhibiting FLT3-ITD clones at relapse.
Clinical data from pivotal venetoclax-based studies reveal that the addition of venetoclax does not significantly improve outcomes or survival for patients with FLT3-ITD. This has spurred the development of novel triplet regimens incorporating FLT3 inhibitors like gilteritinib and quizartinib with venetoclax, showing promising phase I results. Despite this, challenges such as myelosuppression, frequent early marrow assessments, and shortened venetoclax cycles remain common limitations.
INTERVENE TRIAL:
The INTERVENE study focused on evaluating a novel therapeutic regimen in newly diagnosed AML patients aged 60 years and older who were unfit for intensive chemotherapy.
Key Design Features:
- Population:
- Enrolled patients aged 60 years and older with newly diagnosed AML.
- Excluded those with adverse cytogenetics.
- Allowed prior exposure to hypomethylating agents (HMA) for MDS.
- Treatment Plan:
- Utilized midostaurin, a shorter-acting FLT3 inhibitor with a half-life of ~21 hours, to reduce myelosuppression risks.
- Applied a sequential doublet regimen:
- Started with venetoclax and low-dose cytarabine (LDAC).
- Added midostaurin (FLT3 inhibitor) on day 11, after LDAC completion.
- Safety Run-In Phase:
- Conducted with 18 patients, results presented at ASH in 2022.
- Enabled seamless transition to the randomized Phase 2 trial.
- Randomization:
- 2:1 randomization between:
- Midostaurin triplet arm: Venetoclax, LDAC + midostaurin.
- Control arm: Venetoclax + LDAC alone.
- Primary Endpoint: Complete remission (CR) or Complete Remission with Incomplete Hematologic Recovery CRi by the end of cycle 4.
Patient Characteristics:
- Enrolment: 120 patients across 21 centers in Australia and New Zealand.
- Median Age: 74 years, with >25% of triplet arm patients aged ≥80 years.
- FLT3-ITD Mutation: 28% in the triplet arm, 17% in the control arm.
- Other Molecular Profiles: NPM1 and IDH mutations were present in 50% of control arm patients.
Findings:
Efficacy:
- Overall CR/CRi: 72% (triplet arm) vs. 68% (control arm).
- FLT3-ITD Subgroup: CR/CRi: 82% (triplet) vs. 57% (control).
- Median overall survival: 16.6 months (triplet) vs. 8.8 months (control), p=0.10.
- Other Subgroups:
- NPM1-mutated patients showed high response rates in both arms (~93% triplet vs. 84% control).
- Poor outcomes for RAS-mutant patients, with low response rates (~40%) and short survival (~5 months).
- Minimal Residual Disease (MRD):
- FLT3-ITD MRD negativity by cycle 4: 61% (triplet arm).
- By cycle 6: 67% (triplet arm), with no MRD negativity in the control arm (small sample size).
Tolerability and Safety:
- Non-hematological toxicities:
- Higher gastrointestinal AEs (e.g., nausea) in the triplet arm.
- Comparable rates of cardiac events and infections in both arms.
- Myelosuppression:
- No cumulative myelosuppression; median inter-cycle times remained consistent (~35 days) across cycles.
- 30-day mortality: 4% (triplet arm) vs. 10% (control arm).
Conclusion:
In conclusion, the sequential combination of LDAC, venetoclax, and midostaurin delivered in a sequential doublet manner demonstrated improved responses and survival in patients with FLT3 ITD AML. The regimen effectively eradicated FLT3 ITD in the majority of patients, although 44% experienced FLT3 ITD-negative relapses. The treatment maintained comparable inter-cycle times to LDAC and venetoclax alone, with 25% of patients receiving more than 12 cycles, highlighting its prolonged efficacy and safety profile.
10 Year Follow-up of C10603/RATIFY: Midostaurin Versus Placebo Plus Intensive Chemotherapy for Newly Diagnosed FLT3-mutant Acute Myeloid Leukemia Patients Aged 18-60 Years
Speaker: Richard Stone, Monash University, Australia
Key Highlights:
Background of FLT3 inhibitors:
The history of FLT3 inhibitors in AML has been a complex and evolving journey. Preclinical data in the mid-1990s identified mutant FLT3 as a promising therapeutic target in AML. By the mid-2000s, initial single-agent and combination trials demonstrated biological activity but limited clinical success with early FLT3 inhibitors.
However, the CALGB 10603 (RATIFY) trial, initiated in 2007, marked a pivotal moment, establishing midostaurin's role in combination with chemotherapy for FLT3 mutant AML, leading to its approval in the US and Europe in 2017. Subsequent FLT3 inhibitors like gilteritinib and quizartinib were introduced for relapsed or refractory FLT3-mutated AML.
More recently, quizartinib was approved in both the US and Europe for use with chemotherapy in FLT3 ITD mutant AML following the QuANTUM-First study results. These developments highlight the significant progress in targeting FLT3 mutations to improve outcomes for AML patients.
RATIFY Trial:
- The RATIFY CALGB 10603 study was a Phase 3, double-blind, randomized, prospective trial.
- Standard induction chemotherapy included 60 mg of daunorubicin with cytarabine.
- A maintenance phase of one year included either midostaurin or placebo.
- Published results (2017) showed a 22% reduced risk of death with midostaurin.
- Patients were stratified based on FLT3 mutations (TKD or FLT3 ITD, high vs. low allelic ratios).
- The study analyzed event-free survival (EFS), overall survival (OS), and disease-free survival (DFS).
- CR was defined by remission by day 60 or at any point during induction.
Key 10-Year Results:
- OS: Midostaurin group sustained significant survival benefits over placebo (p<0.05), with trends favoring midostaurin across FLT3 subtypes (ITD-high, ITD-low, TKD).
- EFS:
- Significant EFS benefit for midostaurin versus placebo across FLT3
- Most events occurred early (e.g., failure to achieve remission post-induction).
- Impact of Transplant:
- Transplant in first complete remission (CR1) correlated with better outcomes across both arms.
- Patients receiving midostaurin and undergoing transplant in CR1 showed a trend toward improved survival.
- Maintenance Phase:
- No clear survival benefit from midostaurin during maintenance therapy; placebo and midostaurin groups performed comparably.
- Suggests midostaurin suppresses rather than eradicates disease during maintenance.
- Subgroup Insights: NPM1 Mutations: Patients with co-mutated NPM1 benefited significantly from midostaurin in both OS and EFS.
- Gender Differences: OS benefit of midostaurin was not observed in women. Possible explanations include differences in disease biology or treatment responses.
- Late Events: Few relapses occurred after 5 years, but there were AML-related and non-AML-related deaths, including new cancers and complications from late transplants.
Conclusion:
In conclusion, the EFS benefit of adding midostaurin to chemotherapy was sustained over time, though the overall survival benefit was slightly reduced, likely due to aging in both groups. While late events in this study were rare, further investigation into their nature is needed.
A Phase Ib/II Study of Ivosidenib with Venetoclax ± Azacitidine in IDH1-Mutated Hematologic Malignancies: A 2024 Update
Speaker: Jennifer Marvin-Peek, University of Texas
Key Highlights:
Introduction:
Dr. Peek talked about IDH1 mutations which are observed in approximately 7-8% of newly diagnosed AML patients and are also associated with myelodysplastic syndromes and myeloproliferative neoplasms. These mutations are more commonly found in patients with accelerated disease, indicating an increased risk of progression to overt AML. Clinical outcomes suggest that IDH1 mutant AML patients treated with standard regimens generally fare well, with promising responses noted in clinical trials like the VIALE-A and AGILE studies.
Study Overview:
Dr. Peek explained the study in detail. It is a multicentre, investigator-initiated Phase 1 and 2 study to evaluate a triplet regimen combining ivosidenib, venetoclax, and azacitidine. The goal was to determine whether this combination could improve response rates and clinical outcomes in patients with IDH1 mutations.
Eligibility Criteria:
- Adults with a confirmed IDH1 mutation and a diagnosis of either:
- Relapsed/refractory AML, myelodysplastic syndromes (MDS), or myeloproliferative neoplasms (MPN) classified as high risk
- Newly diagnosed AML not eligible for standard induction chemotherapy
- Exclusion: Prior treatment with venetoclax, ivosidenib, or other hypomethylating agents was allowed.
Study Phases: Phase 1 (Dose Escalation):
- Initial cohorts received a doublet combination of ivosidenib and venetoclax.
- Azacitidine was added at dose level 3 to evaluate the triplet regimen.
- Ivosidenib was administered continuously starting at cycle 1, day 15, to minimize differentiation syndrome risk.
- Venetoclax was given for 14 days per cycle, while azacitidine was administered for 7 days.
Objectives:
- Primary Objectives: Assess safety and overall response rate.
- Secondary Objectives: Evaluate overall response, duration of remission, EFS, and MRD negativity by flow cytometry.
Enrolment and Cohort Composition:
- 25 additional patients were added to the Phase 2 cohort with a target enrolment of 60 patients.
- From March 2018 to March 2024, 56 patients initiated treatment:
- 12 with MDS or MPN
- 31 with newly diagnosed AML
- 13 with relapsed/refractory AML
Patient Characteristics:
- Median Age: 69 years
- Gender Distribution: 63% male
- Disease Type: Among patients on a doublet regimen, 50% had relapsed/refractory AML compared to 16% of those on the triplet regimen.
- Risk Stratification: Based on ELN 2022 guidelines, 61% of AML patients had adverse-risk disease.
- Co-mutations:
- NPM1 mutations: Present in 15 patients
- Signalling mutations (FLT3-ITD or RAS): Found in 13 patients
- TP53 mutations: Found in 5 patients
- Prior Treatments: 30 patients had received prior therapies, including 3 patients who had previously been treated with an IDH1
Key Findings:
Efficacy:
- ORR: 94%; composite complete remission (CR/CRi) rate: 93%.
- MRD negativity: Achieved in 76% of patients.
- Median time to best response: 53 days.
- Transition to stem cell transplant (SCT): 22 patients (39%).
Survival outcomes:
- Median OS: Not reached.
- 3-year OS: 71%.
- Median EFS: 50.4 months.
- Median DoR: 43.4 months (censored at SCT).
Subgroup Analysis: Mutational Subgroups:
- NPM1 co-mutations: No significant impact on OS/DOR.
- TP53 mutations: Poorer outcomes.
- RAS mutations: Trend toward worse outcomes, not statistically significant.
Triplet therapy showed longer remission duration but comparable OS/EFS.
Patients undergoing SCT had a 3-year OS of 95%.
Safety and Tolerability:
- Most adverse events (AEs) were grade 1/2.
- Grade 3+ AEs: 27% (e.g., tumor lysis syndrome, QTc prolongation, differentiation syndrome).
- Differentiation syndrome cases (n=4) were successfully managed with steroids/supportive care.
- Low rates of neutropenic fever, attributed to standard prophylaxis and minimal added myelosuppression from ivosidenib.
Conclusion:
Triplet therapy with Ivosidenib, Venetoclax, and Azacitidine demonstrated safety, efficacy, and durable responses with a median follow-up of three years. Key outcomes included:
- Composite Complete Remission Rate: 93%
- Median DoR: 43 months
- Median OS: Not yet reached
Long-Term Survival Outcomes and Cytogenetic/Molecular Patterns of Relapse in Adults with FLT3-Mutated AML Receiving Frontline Triplet Therapy with a Hypomethylating Agent, Venetoclax and FLT3 Inhibitor
Speaker: Nicholas Short, University of Texas
Key Highlights:
Study Background:
- FLT3 Mutations in AML: Present in ~30% of newly diagnosed AML cases, FLT3 mutations (ITD or TKD) predict poor outcomes with high relapse rates.
- Current Challenges: The standard of care for older/unfit patients (hypomethylating agent + venetoclax) is less effective in FLT3-mutated AML, with a median survival of ~9.9 months in VIALE-A.
- Triplet Therapy Rationale: Adding a FLT3 inhibitor (gilteritinib or quizartinib) aims to overcome FLT3-driven relapse and improve survival.
Study Design:
This study was a retrospective analysis of adults with newly diagnosed FLT3-mutated AML treated with a triplet regimen of a hypomethylating agent, Venetoclax, and a FLT3 inhibitor on clinical trials at a single institution.
Key Inclusion & Exclusion Criteria:
- Inclusion: Patients with any established FLT3-activating mutation.
- Exclusion: Patients with treated secondary AML (clinical course resembling relapsed/refractory AML).
Objectives:
- Evaluate prognostic factors and long-term survival.
- Assess clonal evolution patterns at relapse.
- Determine post-relapse outcomes.
Treatment Plan Highlights:
- Patients were enrolled across various frontline clinical trials with slight variations in drug doses and durations.
- For most patients, the regimen included Azacitidine, Venetoclax, and Gilteritinib with strategies to mitigate myelosuppression:
- Day 14 bone marrow evaluations during cycle 1, followed by holding Venetoclax and FLT3 inhibitors for patients in marrow remission.
- Reduced doses of Azacitidine and Venetoclax during consolidation cycles.
- Gilteritinib dose adjusted to 80 mg/day instead of the standard 120 mg/day.
Key Findings:
- Response Rates:
- 93% CR/CRi rate; 82% achieved full CR.
- 81% of evaluable patients achieved MRD negativity.
- Survival Outcomes:
- Median OS: 38.5 months.
- 3-year OS: 52%.
- Median RFS: 28.8 months.
- FLT3-ITD patients: Median OS of 28.1 months vs. 9.9 months in VIALE-A.
- FLT3-TKD patients: Median OS of 39.3 months vs. 19.2 months in VIALE-A.
- Subgroup and Predictive Factors:
- Age: No significant survival difference between those ≥75 and <75 years old.
- Mutation Co-occurrences: Outcomes were worse for patients with baseline RAS pathway mutations (3-year OS: 22%) or triple mutations (FLT3-ITD, NPM1, and DNMT3A).
- Relapse Patterns:
- 65% of relapses were FLT3-negative, indicating effective FLT3
- RAS pathway mutations emerged in 24% of relapses.
- Post-Relapse Outcomes:
- Median OS after relapse: 6.1 months.
- FLT3-positive relapses had worse survival (median OS: 1.6 months) compared to FLT3-negative relapses (10.1 months).
- Role of SCT: 41% underwent SCT; transplant did not significantly improve survival outcomes, likely due to older patient cohorts and higher transplant-related mortality (20%).
Conclusion:
- The triplet regimen of a hypomethylating agent, Venetoclax, and a FLT3 inhibitor achieved durable remissions and improved survival in FLT3-mutated AML.
- Median overall survival was 28.1 months for FLT3-ITD and 39.3 months for FLT3-TKD mutations, outperforming historical doublet therapy.
- About 65% of relapses were driven by FLT3-negative clones, emphasizing the effectiveness of FLT3 inhibitors in eliminating FLT3-mutated subclones but highlighting the need for strategies to prevent FLT3-negative relapses.
- Worse outcomes were observed in patients with baseline RAS pathway mutations, which also contributed to approximately 25% of relapses, underscoring their role in resistance.
Gilteritinib Results in Higher Remission and Transplant Rates Than Midostaurin but Does Not Increase the Post-Induction Mutational MRD Negative Rate: Results of the Phase 2 Randomized Precog 0905 Study in Newly Diagnosed FLT3 Mutated AML
Speaker: Selina Luger
Key Highlights:
Study Background:
FLT3 Mutations in AML: Commonly associated with poor prognosis, high relapse rates, and inferior survival outcomes.
Rationale: Gilteritinib, a second-generation FLT3 inhibitor, has demonstrated efficacy in relapsed/refractory AML. This study aimed to compare it to midostaurin (a first-generation inhibitor) in newly diagnosed FLT3-mutated AML, focusing on remission and mutational measurable residual disease (MRD) negativity.
PrECOG 0905 Study:
- The PrECOG 0905 trial was an open-label, randomized phase 2 study for patients with newly diagnosed FLT3-mutated AML.
- Participants received daunorubicin (90 mg/m² for 3 days) and cytarabine (100 mg/m² for 7 days) combined with either gilteritinib (120 mg daily, days 8–21) or midostaurin (50 mg twice daily, days 8–21).
- Those achieving complete remission (CR) or CR with incomplete count recovery (CRi) proceeded to consolidation with high-dose cytarabine and their assigned FLT3
- Primary and Secondary Objectives:
- The primary objective was to improve the composite CR rate in FLT3 mutation-negative populations, comparing gilteritinib to midostaurin.
- Secondary outcomes included MRD negativity, survival, and relapse-free survival.
- Eligibility and Screening:
- Central lab prescreening confirmed FLT3 (ITD/TKD) and NPM1 mutation status for eligibility.
- Initially open to patients aged 18–65, later amended to include those up to age 70 with adequate organ function.
- Patient Enrolment: From Nov 2019 to Nov 2022, 722 patients were screened across 37 centres, with 180 randomized and 177 treated.
Patient Characteristics:
- Median Age: 54 years; 27.7% were over age 60.
- Gender: 58% female.
- Race/Ethnicity: 77% White, 8.5% Black, 8.5% Hispanic
- FLT3 Mutation Status: Majority had ITD mutations with a high signal ratio.
- NPM1 Mutation: Similar distribution between both arms.
- Risk Profile:
- 11% had high-risk ELN.
- 3% had adverse karyotypes.
- Treatment Allocation: 177 eligible and treated patients:
- 90 received gilteritinib.
- 87 received midostaurin.
Key Findings:
Efficacy:
- CRi Rate: Higher with gilteritinib (85.6%) than midostaurin (72.4%, p=0.03).
- FLT3-Negative CRi Rate Post-Induction:
- Gilteritinib: 40%.
- Midostaurin: 47.1% (not significant).
- MRD by Flow Cytometry Post-Induction: Slightly higher with gilteritinib (64.4% vs. 59.8%; not significant).
- FLT3 Mutation Clearance After First Consolidation Cycle:
- Gilteritinib: 83% cleared FLT3
- Midostaurin: 44% (p<0.05).
Transplant Rates: Gilteritinib significantly increased the proportion of patients proceeding to transplant in first remission (60% vs. 46% for midostaurin; p=0.04).
Subgroup Analysis: No statistically significant differences in FLT3-negative CRC by FLT3 mutation subtype (ITD or TKD), NPM1 co-mutation status, or triple-mutated status (FLT3-ITD, NPM1, and DNMT3A).
Adverse Events: No significant differences in treatment-related toxicities or induction-related deaths.
Conclusion:
- Induction therapy with daunorubicin, cytarabine, and gilteritinib achieved a greater than 86% complete remission with incomplete hematologic recovery (CRi) rate in newly diagnosed FLT3-mutated AML patients up to age 70, with no treatment-related deaths.
- Compared to midostaurin, gilteritinib increased the CRc rate but did not significantly improve CRc rates in FLT3 mutation-negative patients. Multivariable regression identified factors such as FLT3 ITD allelic ratio, NPM1 mutation status, WBC count, and hemoglobin levels at diagnosis as impacting CRc outcomes in FLT3-negative patients.
- Notably, more FLT3 mutation-positive patients on gilteritinib achieved MRD negativity after post-consolidation 1 reassessment and were more likely to proceed to CR1 transplant.
Prospective Study of Avapritinib in Patients with MRD-Positive or Relapsed/Refractory Core-Binding Factor Acute Myeloid Leukemia (CBF-AML) with KIT Mutations
Speaker: Xueqing Dou, The First Affiliated Hospital of Soochow University, China
Key Highlights:
Introduction:
Core-binding factor (CBF) AML is characterized by two main genetic abnormalities: t(8;21) translocation and inv(16) or t(16;16) chromosomal changes. These result in the formation of the RUNX1-RUNX1T1 and CBFB-MYH11 fusion genes, respectively.
Approximately 25–35% of patients with CBF AML have co-occurring KIT gene mutations. While most patients respond well to chemotherapy, with OS and RFS) rates ranging from 60–80%, around 30% experience relapse. KIT mutations are associated with a higher relapse rate and poorer prognosis in these patients.
Avapritinib, a highly selective inhibitor of KIT and PDGFRA mutants, blocks kinase activity, inhibiting tumor cell proliferation and survival. Although approved for specific conditions like PDGFRA-mutant gastrointestinal stromal tumors (GIST) and systemic mastocytosis, its efficacy in KIT-mutated CBF AML remains unclear.
Study Overview:
This was a prospective, multi-center, single-arm clinical trial to evaluate the efficacy and safety of avapritinib for treating CBF AML with KIT mutations.
The aim was to potentially expand the indications of avapritinib to include AML.
- Patient enrolment: Participants aged 15 years and older with CBF AML and KIT mutations were included in one of two cohorts:
- Cohort 1: Patients in haematological CR with molecular MRD positivity. MRD positivity was defined as detectable RUNX1-RUNX1T1 or CBFB-MYH11 transcripts via qPCR.
- Cohort 2: Patients with relapsed or refractory disease at the time of enrolment.
- Treatment Protocol: Patients received avapritinib at a daily dose of 100–200 mg. Combination with other treatments was permitted.
- Endpoints:
- Primary Endpoint: ORR, with response in Cohort 1 defined as a reduction in MRD levels by ≥1 log.
- Secondary Endpoints: Composite CR rate, safety, and survival outcomes.
Patient Characteristics:
The study enrolled 40 eligible patients from Nov 2022 to Oct2024 across nine medical centres in China.
- MRD Status:
- 28 patients were MRD positive.
- 12 patients were relapsed or refractory at enrolment.
- Fusion Status:
- 31 patients had the RUNX1-RUNX1T1
- No patients had the CBFB-M1H11
- KIT Mutations:
- Four types of KIT mutations were observed.
- The most common was the 816 mutation.
- Other mutations included the 822 mutation, compound mutations, and other variants.
Results:
Median response time: 2 cycles (RUNX1-RUNX1T1), 3 cycles (CBFB-MYH11).
MRD-Positive Cohort (n=28):
- After 1 Cycle:
- Response rate: 17.8%.
- MRD-negative conversion: 14.3%.
- After ≥3 Cycles:
- Response rate: 81.3%.
- MRD-negative conversion: 50%.
- Overall: MRD-negative conversion in 42.9% (12/28).
Relapsed/Refractory Cohort (n=12):
- Composite CR rate: 41.7%.
- ORR: 50%.
Among responders:
- 3 underwent allogeneic HSCT with sustained MRD negativity.
- 3 maintained remission for 1–9 months but eventually relapsed.
Adverse Events:
- Hematologic Toxicities: Grade 3–4 myelosuppression in ~20% (leukopenia, thrombocytopenia).
- Non-Hematologic Toxicities: Facial edema, liver enzyme elevation, and graying hair. No central nervous system (CNS) bleeding was observed despite concerns about avapritinib crossing the blood-brain barrier.
Conclusion:
- Avapritinib demonstrated efficacy in MRD reduction and inducing CR in relapsed/refractory KIT-mutated CBF-AML.
- Longer treatment cycles were associated with improved responses (≥2–3 cycles recommended).
- RUNX1-RUNX1T1 cases responded faster than CBFB-MYH11 (median 2 vs. 3 cycles; statistically significant).
- Safety: Generally tolerable, with expected myelosuppression and mild non-hematologic toxicities.
- Clinical Implications: Avapritinib shows potential as a targeted therapy in MRD-positive or relapsed/refractory KIT-mutated CBF-AML. Patients with sustained responses could proceed to HSCT for potentially curative outcomes.
ASH Annual Meeting and Exposition, 7-10 December 2024, San Diego, California