ERS 2024: Circulating Biomarkers in Severe PAH
Speaker- Laurent Savale
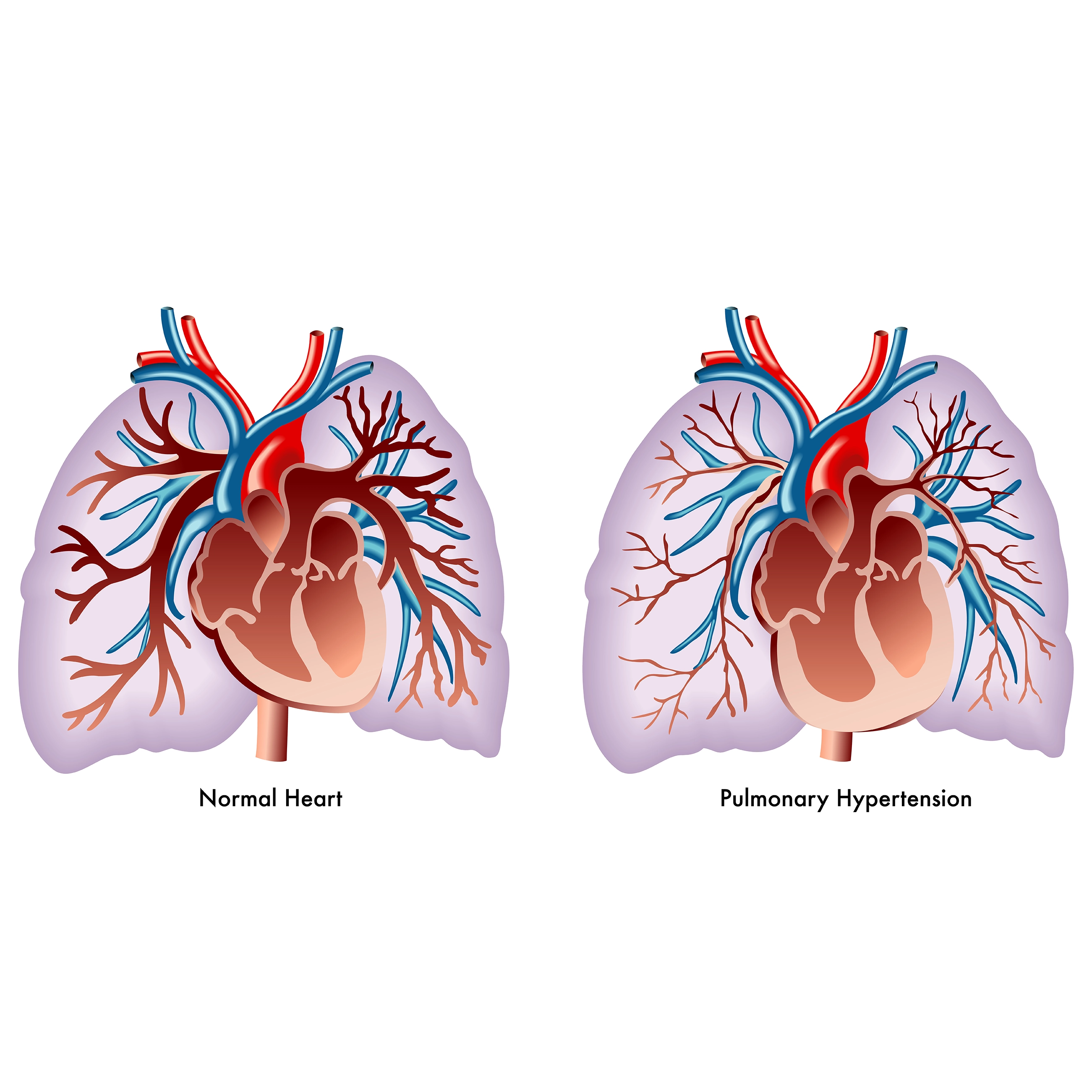
The severity of pulmonary hypertension is determined by multiple factors, including the extent of pulmonary vascular lesions, which contribute to a maladaptive right ventricular phenotype in patients. Additionally, multisystemic organ damage caused by right ventricular failure plays a significant role in disease progression. External factors also influence the course of pulmonary arterial hypertension (PAH), such as the subtype of PAH, existing comorbidities, patient sex, and genetic mutations. Hence, it is important to use diagnostic tools to assess the severity of the disease, enabling the early detection of severe PAH, which can be defined by one year mortality risk of >20%, according to the latest guidelines. Several challenges persist in improving the survival of patients with severe PAH. These include evaluating the effectiveness of novel drugs targeting the activin pathway, identifying at-risk patients for better transplant optimization, and enhancing the monitoring and management of those admitted to intensive care units (ICUs) with acute decompensated PAH.
Biomarkers offer a valuable approach to assessing the severity of PAH. Several methods exist for identifying novel biomarkers in this condition. One approach is through omics studies, which involve large-scale screening of biomarker panels without relying on predefined hypotheses. Another method evaluates specific biomarkers based on established hypotheses, such as those related to endothelial dysfunction, inflammation, and organ damage. In recent years, numerous biomarkers have been identified. However, a significant gap remains between discovery of biomarkers and their integration into clinical practice to assess disease severity of the patients. The available biomarkers in routine clinical practice assess patients with end-stage pulmonary hypertension. These include biomarkers that indicate myocardial stress and end-organ failure due to right heart failure. Only a small proportion of identified biomarkers are applicable for routine clinical use. There are neither diagnostic biomarkers available for the early detection of PAH, nor predictive biomarkers that can forecast treatment responses in PAH patients. This represents a crucial area for further research and development.
The development of new treatment targeting the activin pathway and the growing understanding of its role in the pathophysiology of PAH provides a valuable opportunity to evaluate biomarkers related to this pathway. These biomarkers can include the ligands involved in the activin pathway and also its modulators, such as inhibitors like follistatin, inhibin alpha, and Follistatin-like 3 (FSTL3). In a cohort of patients with idiopathic, heritable, or anorexigen-associated PAH, Activin A and B levels were compared. The levels of these ligands in the activin pathway were observed to be significantly elevated in patients with PAH. In terms of the inhibitors of the activin pathway, there was a marked decrease in circulating levels of inhibin alpha, while follistatin and FSTL3 levels were notably increased in these patients. These findings align with the observations noted in the pulmonary vascular lesions of PAH patients, where an overactivation of the activin pathway has been noted in both the endothelial and smooth muscle cells, and in patients who underwent lung transplantation for end-stage PAH. Additionally, this overactivation is associated with nuclear accumulation of phosphorylated Smad2/3 proteins, indicating the overexpression of activin pathway components.
When analyzing the prognostic value of the Activin A and FSTL3 biomarkers in patients with PAH, it was observed that both biomarkers were significantly associated with patient survival at baseline and after initiating first-line PAH therapies. Patients with elevated Activin A and FSTL3 levels had a distinct end-stage PAH phenotype with a lower cardiac index, higher right atrial pressure, and reduced six-minute walk distance(6MWD). The prognostic value of Activin A and FSTL3 remained significant even after adjusting for other non-invasive parameters, including functional class, 6MWD, and N-terminal pro-b-type natriuretic peptide (NT-proBNP). This indicates that these biomarkers provide additional prognostic value beyond traditional clinical measures. Furthermore, when determining the optimal threshold levels of Activin A and FSTL3, a clear distinction was found between patients with favorable long-term outcomes and those with worse prognoses. Patients with lower circulating levels of these two biomarkers demonstrated better outcomes, while those with elevated levels developed more severe pulmonary hypertension and had poorer outcomes. These findings were validated in an independent cohort from the United Kingdom, further supporting the reliability of Activin A and FSTL3 as prognostic biomarkers for PAH.
In studies utilizing non-predefined hypothesis approaches, with using digital spatial transcriptomic signatures, researchers identified significant activation of the gene coding for the TGF-β pathway in the pulmonary vascular lesions of PAH patients, particularly in plexiform lesions. A study conducted in a Canadian cohort of PAH patients further investigated the prognostic value of over 300 biomarkers through an omics platform. This study identified six proteins independently associated with mortality and lung transplantation, even after adjusting for risk stratification tools. One key finding from the study was the inclusion of FSTL3 among the six proteins, with higher levels observed in patients exhibiting maladaptive right ventricular failure and were associated with poorer survival outcomes. Additionally, FSTL3 was overexpressed not only in the lungs but also in the hearts of animal models with PAH, further supporting its role in disease progression and right heart dysfunction. An important clinical challenge is the management of PAH patients admitted to ICUs for acute decompensated PAH. These patients often face a high short-term mortality risk, but rescue therapies such as extracorporeal membrane oxygenation (ECMO) and urgent transplantation may offer potential lifesaving interventions. Hence, early identification of patients developing refractory right heart failure is crucial in this setting. To date, data on patients with acute right heart failure admitted to ICUs have been mostly retrospective. To summarise, cardiac biomarkers such as B-type natriuretic peptide (BNP), NT-proBNP, and troponin, along with inflammation and end-organ failure biomarkers, have been commonly associated with patient survival. However, prospective data are needed further to improve the severity assessment and management of these critically ill patients. Preliminary findings from the ProPULS study, a prospective cohort of ICU-admitted PAH patients, offer insight into short-term outcomes in these patients. Blood samples were collected at baseline, day three, and day seven, with the primary endpoint being occurrence of death, ECMO, or transplantation within three months post-admission. Of the 26 patients who experienced an event within 3 months of admission, prognostic value of various biomarkers affecting activin pathway, inflammation pathway and of myocardial stress and secondary organ damage was assessed. At baseline, two biomarkers—growth differentiation factor 15 (GDF-15) and uric acid—were associated with short-term outcomes. However, when analyzing the evolution of biomarkers over the first three days, additional markers emerged as predictive of refractory right heart failure. FSTL3, in particular, appeared to be a promising biomarker for assessing the severity of PAH patients in ICUs with acute right heart failure. Detailed analysis of biomarker levels of PAH patients in ICU further supports the potential utility of these markers in guiding clinical decisions.
Novel treatments such as Sotatercept, targeting the activin pathway, have shown encouraging results in modifying key biomarkers, including NT-proBNP, FSTL3, Bone Morphogenetic Protein 9 (BMP9), and BMP10, all of which are relevant to the pathophysiology of severe and end-stage PAH. In phase II and III trials, Sotatercept had a positive effect on NT-proBNP, sometimes causing a rapid and significant decrease in these levels, especially in severe PAH. Additionally, the ongoing ZENITH trial aims to assess efficacy of Sotatercept in patients with high risk PAH on maximal drug therapy, particularly of functional classes III and IV, with results expected later. In an exploratory analysis of patients enrolled in Phase II and III trials of Sotatercept, more than 3,000 biomarkers were analysed using the omics involving 17 patients on placebo and 14 on Sotatercept. The results revealed 52 significant differentially expressed proteins between the treatment and placebo groups. These included cardiovascular biomarkers such as NT-proBNP and others such as FSTL3, BMP9, BMP10, inflammation-related markers, and those related to metabolism of lipids, nucleotides, and amino acids. While Sotatercept shows promise, the exact implications of these biomarker modulations—whether positive or negative—remain unclear. Further investigation is necessary to determine whether these changes directly result from the drug or reflect improvements in the underlying pulmonary hypertension.
In conclusion, end-stage PAH is a complex disease, and to adequately reflect the full spectrum of the disease, a multiple biomarker approach is needed. The serum levels of Activin A and FSTL3 appear to be promising prognostic biomarkers in PAH, relevant at different stages of disease severity. These biomarkers provide valuable insights into the progression of PAH and may offer prognostic value in the early and more advanced stages of the disease.
European Respiratory Society Congress 2024, 7–11 September, Vienna, Austria