CREDENCE: Impact of Canagliflozin on Clinical Outcomes across a Broad eGFR Range
1 Sep, 20
Background
The CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) trial demonstrated that canagliflozin reduced renal and cardiovascular (CV) events in people with type-2 diabetes mellitus (T2DM) and substantial albuminuria.
Aim
To determine whether the impact of canagliflozin on clinically important kidney, CV, and safety outcomes would remain consistent across a broad range of estimated glomerular filtration rate (eGFR), including the lower eGFR range of 30–45 ml/min per 1.73m2
Patient Profile
- Patients with T2DM (age ≥ 30 years), glycated hemoglobin (HbA1c) 6.5%-12.0%, eGFR of 30–<90 ml/min per 1.73 m2, urinary albumin-creatinine ratio (UACR) >300–5000 mg/g (n=4401)
- All patients were treated with stable maximum labeled or tolerated dose of angiotensin converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) for ≥4 weeks before randomization
Methods
Study Design
- Secondary/ Post hoc analysis of the CREDENCE trial
- CREDENCE was a randomized, double-blind, placebo-controlled, multicenter clinical trial
Treatment Strategy
- Patients underwent 2-week single-blind placebo run-in period before randomization
- Based on the baseline eGFR patients were stratified into categories of 30 to <45, 45 to <60, and 60 to <90 ml/min per 1.73 m2
- Patients were randomized to canagliflozin (100 mg) or placebo
Follow-up
- Median duration; 2.62 years
Outcomes
Primary Outcome
- A composite of end stage kidney disease [(EKSD); chronic dialysis for ≥30 days, kidney transplantation, or eGFR <15 ml/min per 1.73 m2 sustained for ≥30 days, doubling of serum creatinine from baseline values, sustained for ≥30 days, or death due to renal or cardiovascular disease (CVD)]
Secondary Outcomes
- A composite of ESKD, doubling of serum creatinine, or renal death
- ESKD
- Doubling of serum creatinine
- An exploratory composite of initiation of RRT (renal replacement therapy; initiation of chronic dialysis for ≥30 days or receipt of a kidney transplant) or renal death
Other Efficacy Outcomes
- A composite of CV death or hospitalization for heart failure (HF)
- A composite of CV death, myocardial infarction (MI), or stroke
- Hospitalization for HF
Safety Outcomes
- Adverse events and serious adverse events, amputation, fracture, osmotic diuresis, and volume depletion
Results
- CREDENCE trial was stopped at interim analysis considering the efficacy of canagliflozin
- Mean age of the study population was 63 years, 34% were female, 67% were white, and 20% were Asian. The mean HbA1c was 8.3% and mean BP was 140/78 mmHg, the mean baseline eGFR was 56.2 ml/min per 1.73 m2 and median UACR was 927 mg/g. Nearly 50% patients had a history of CVD.
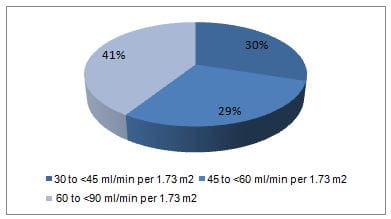
- At screening, substantial proportion of patients had eGFR of 30 to <45 ml/min per 1.73 m2 (Figure 1).
Figure 1: Proportion of patients as per the eGFR categories
- Canagliflozin brought about a significant 30% reduction in the primary composite outcome, with consistent benefits across all eGFR subgroups (hazard ratio [HR], 0.70; 95% confidence interval [CI], 0.59 to 0.82; all P interaction 0.11) compared to placebo. (Figure 2)
Figure 2: The incidence of Primary composite outcomes in the study groups
- The effect of canagliflozin with regards to renal composite outcome of ESKD, doubling of serum creatinine, or renal death (HR, 0.66; 95% CI, 0.53 to 0.81), ESKD, doubling of serum creatinine, and the composite of initiation of RRT or renal death was also consistent across all eGFR subgroups.
- Patients in lower eGFR subgroup (30 to <45 ml/min per 1.73m2) experienced larger absolute benefits for renal outcomes with a significant 29% relative reduction in renal outcomes (HR, 0.71; 95% CI, 0.53 to 0.94) compared to placebo.
- The benefits of canagliflozin in terms of reduced CV death or hospitalization for HF; the composite of CV death, MI, or stroke; and hospitalized HF, remained consistent across all eGFR subgroups (all P values for interaction> 0.25). In particular, patients with screening eGFR of 30 to <45 ml/min per 1.732 had a 31% reduction in the composite of CV death or hospitalization for HF (HR, 0.69; 95% CI, 0.50 to 0.94) compared to placebo.
- Patients treated with canagliflozin had fewer adverse events (P interaction =0.40) and fewer serious adverse events (P interaction = 0.15), with consistent results across all eGFR subgroups. The incidence of adverse events including fractures and amputations did not differ significantly between canagliflozin and placebo treated patients.
- Although the incidence of volume depletion and osmotic diuresis events differed across eGFR subgroups (P interaction =0.01 and 0.03, respectively), the overall incidence was not frequent in canagliflozin-treated patients,
- There were no unexpected safety concerns with regards to canagliflozin amongst patients with screening eGFR of 30 to <45 ml/min per 1.73 m2.
- Canagliflozin treatment was associated with an acute eGFR drop followed by relative stabilization of eGFR loss, across all eGFR subgroups (all p<0.001), with a least drop in those with screening eGFR of 30 to <45 ml/min per 1.73 m2 per year (initial drop of 2.03 ml/min per 1.73 m2).
- In patients with a baseline eGFR 30 – 45 m;/min per 1.73 m2, it was observed that post stabilization, decline in eGFR in the canagliflozin-treated patients was slower vs. placebo group (–1.72 vs. –4.33 ml/min per 1.73 m2; between-group difference 2.61 ml/min per 1.73 m2). These results were true for all eGFR categories.
- Patients treated with canagliflozin had improved total eGFR slope, the combined effect of the acute effect and chronic change in slope from baseline to week 130, overall and in every eGFR subgroup (all P<0.001), without any variation across eGFR subgroups (P heterogeneity =0.71).
- Canagliflozin offered greater absolute benefits for all renal outcomes other than dialysis, transplantation, or renal death over placebo amongst patients with lower screening eGFR. These effects were consistent across all eGFR subgroups.
Conclusions
- Canagliflozin safely reduced the risk of renal and CV events, with consistent results across eGFR subgroups, including the subgroup initiating treatment with an eGFR of 30 to <45 ml/min per 1.73 m2.
- Absolute benefits for renal outcomes were greatest in subgroups with lower eGFR. Canagliflozin attenuated the chronic decline in eGFR over time by 60% and 65%in those with initial eGFR 30 to <45 and 45 to <60 ml/min per 1.73 m2, respectively.
- Patients in the low eGFR subgroups experienced no major safety concerns
- The findings support expansion of treatment with canagliflozin to T2DM patients with an eGFR of 30 to <45 ml/min per 1.73m2.
J Am Soc Nephrol. 2020;31: 1128–1139.