Introduction
The patients with Asthma can be well managed by Inhaled corticosteroids e.g. beclometasone dipropionate [BDP], budesonide, fluticasone propionate which offer prolonged disease control. The addition of a long-acting inhaled ?2-agonist i.e. formoterol fumarate [formoterol] and salmeterol are alternatives used as add on for the patients who do not achieve maximum control with inhaled corticosteroids alone.
Method
In mild to moderate or moderate to severe asthma adult patients, the fixed-combination BDP/ formoterol HFA pMDI was assessed in 4 double-blind, randomized, multicentre trials. In one 12-weeks study, BDP/ formoterol HFA pMDI 100?g/6?g twice daily was compared with BDP 500?g twice daily intervened via a CFC pMDI. In another 24-weeks study, BDP/formoterol HFA pMDI 200?g/12?g twice daily was compared with BDP 500?g twice daily utilized by a CFC pMDI alone or in combination with formoterol 12?g twice daily intervened via a dry powder. Then 12-weeks study was third study which compared BDP/formoterol HFA pMDI 200?g/12?g twice daily with fluticasone propionate/salmeterol 250?g/50?g twice daily administered via pMDI and a fourth study compared BDP/ formoterol HFA pMDI 200?g/12?g twice daily with budesonide/formoterol 400?g/12?g twice daily managed by dry powder inhaler for 12 weeks.
Results
Therapeutic Efficacy
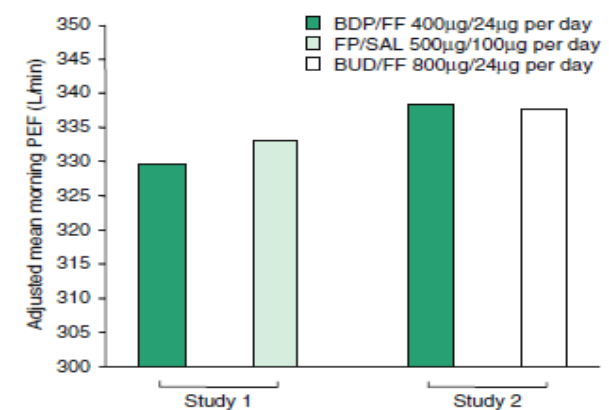
In all 4 studies the primary endpoint was the mean morning pre-dose peak expiratory flow (PEF) analyzed over the last 14 days of treatment. 3 studies evaluated the BDP/formoterol HFA equipotency versus the comparator If the lower margin of the 95% CI for the between-group difference in morning PEF was greater than –20 L/min. In the treatment population 227, 216, 395 and 643 patients were involved. BDP/formoterol HFA pMDI 400?g/24?g per day was similar to fluticasone propionate/ salmeterol pMDI 500?g/100?g per day, and to budesonide/formoterol 800?g/24?g per day intervened as a dry powder inhaler considering the morning PEF in patients with moderate to severe asthma. The mean morning PEF values for the final 14 days of both studies are displayed in Figure 1.
Patients in one study (study 1) received BDP/FF HFA pMDI 400?g/24?g per day or fluticasone propionate/ salmeterol (FP/SAL) 500?g/100?g per day administered via pMDI (n = 227), (p < 0.001) whereas those in the other study (study 2), received BDP/FF HFA pMDI 400?g/24?g per day or budesonide/formoterol (BUD/FF) 800?g/24?g per day administered via DPI (n = 216) for 12 weeks (p < 0.001). All study drugs were fixed combination formulations and the daily dosage was administered as a divided dose twice daily. Morning PEF values were averaged over the last 14 days of treatment.
DPI = dry powder inhaler; HFA = hydofluoroalkane; pMDI= pressurized metered-dose inhaler.
Tolerability
The tolerability of BDP/ formoterol HFA pMDI were retrieved from the clinical studies mentioned above and from a double-blind, randomized, three-way crossover, placebo-controlled tolerability trial. The later study investigated the patients with asthma taking regular treatment with the combination, doses more than the recommended dose of BDP/formoterol are lesser as well tolerated as formoterol. Additionally, the study examined the concomitant intervention of BDP may worsen the reduction in serum potassium levels observed with high doses of formoterol (previously analyzed for other fixed combinations of formoterol and inhaled corticosteroids). In the current study, patients (n = 18) with moderate to severe asthma (50–80% predicted FEV1) underwent open-label maintenance treatment with twice-daily BDP/formoterol HFA pMDI 200?g/12?g were managed ten doses of BDP/ formoterol 100?g/6?g, formoterol 6?g or placebo on each of days 14, 21 and 28 as an addition to the morning administered dose. The primary endpoint was the serum potassium level evaluated over a 12-hour period, meanwhile the secondary endpoints involved corrected QT (QTc) interval and plasma lactate levels along with vital signs. The findings are available as abstracts and/or posters and as data on the file.
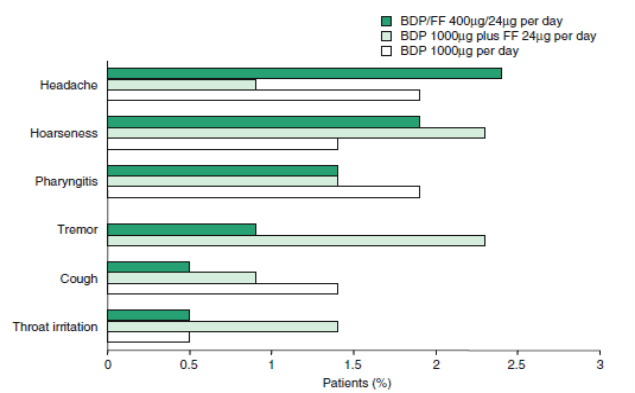
BDP/formoterol HFA/pMDI was normally well tolerated in patients with moderate to severe asthma in a 24-week study. The occurrence of treatment related adverse events was 12.3% in BDP/ formoterol HFA pMDI 400?g/24?g per day receivers, 17.3% in BDP CFC pMDI 1000 ?g plus formoterol dry powder inhaler 24 ?g/day taking patients and in 15.0% in BDP CFC pMDI 1000?g/day intervened patients. Only one serious adverse event (oesophageal candidiasis) in the BDP/formoterol group was decided as the treatment related. The treatment-related adverse events with an incidence of ≥1.0% in any treatment group are depicted in Figure 2.
Incidence of treatment-related adverse events that occurred in ≥1.0% of BDP/FF HFA pMDI, beclometasone (BDP) plus formoterol (FF) or BDP monotherapy recipients. In a randomized, double-blind, multicentre phase III trial, patients (n = 643) received BDP/FF 400?g/24?g per day administered via pMDI or BDP 1000?g per day administered via a CFC pMDI either alone or in combination with formoterol 24?g per day administered via a DPI for 24 weeks. All study drugs were administered as a divided dose twice daily. No recipients of BDP monotherapy reported tremor.
CFC = chlorofluorocarbon; DPI = dry powder inhaler; HFA = hydofluoroalkane; pMDI = pressurized metered-dose inhaler.
BDP/formoterol HFA pMDI found to be efficacious and well tolerated in patients with asthma.
Conclusion
In Germany BDP/formoterol HFA pMDI has been validated for clinical practice and expecting confirmation from other European countries. The fixed-combination BDP/formoterol HFA pMDI is efficacious than BDP intervened alone in asthma patients when mean morning PEF was considered. Moreover, BDP/formoterol HFA pMDI is equipotent with BDP and formoterol utilized via separate inhalers, with fixed-combination budesonide/formoterol, and with fixed-combination fluticasone propionate/salmeterol. BDP/formoterol HFA pMDI causes rapid bronchodilation. BDP/formoterol HFA pMDI is well tolerated in patients with asthma.
Adapted from: Sohita Dhillon and Gillian M. Keating. Beclometasone Dipropionate/ Formoterol; In an HFA-Propelled Pressurized Metered-Dose Inhaler. Drugs. 2006;66(11):1475-83; discussion 1484-5.