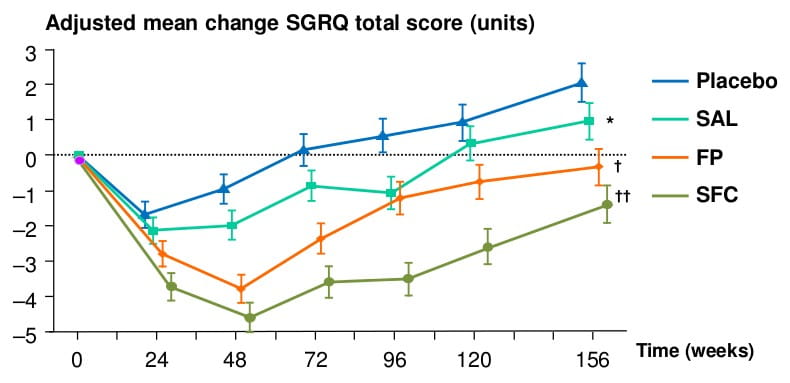
Patients receiving SFC had better quality of life (SGRQ total score) at the end of the study than at the beginning
TORCH (Towards A Revolution in COPD Health) Study
29 Sep, 11
TORCH
Towards a Revolution in Copd Health
TORCH : A Landmark Study
- First Pharmacological intervention to show evidence in impacting mortality after LTOT and smoking cessation
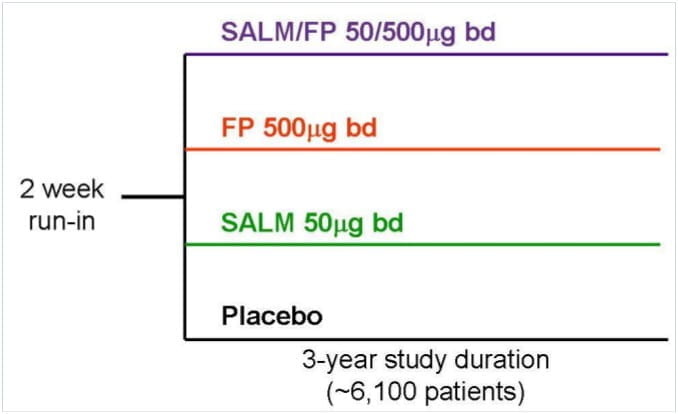
- Large study population (n ∼ 6,000 )
- Results reconfirm the role of ICS in severe COPD
Primary Objective
- The effect of SFC 50/500 µg vs placebo on all-cause mortality over 3 years in patients with moderate-to-severe COPD
Secondary Objectives
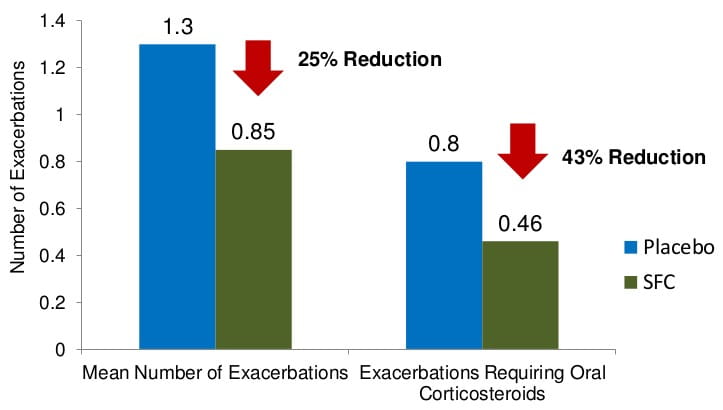
- The effect of SFC 50/500 ?g on the rate of moderate and severe exacerbations
-
The effect of SFC 50/500 ?g on quality of life (SGRQ)
Study Design
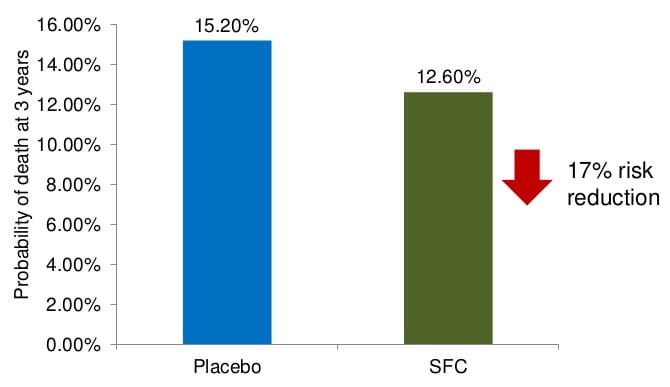
Results: Mortality
Results: Exacerbations
Quality of Life Over 3 Years
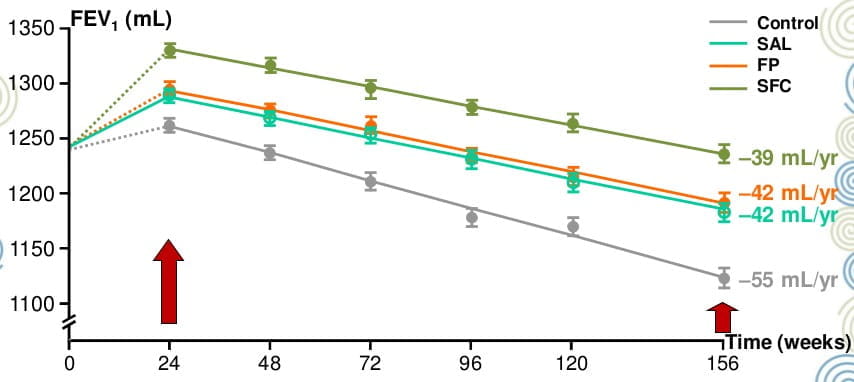
Rate of Decline in Lung Function Over 3 Years
SFC significantly reduced the rate of decline in lung function compared with placebo (39 mL/year vs 55 mL/year, difference 16 mL/year p<0.001)
”...SFC decreas[ed] the excess FEV1 decline attributable to COPD by approximately half.”
“...halving the excess decline in FEV1 is likely to be clinically important...”
Error bars represent 5% and 95% confidence intervals
The Mortality Benefit with SFC vs Placebo is Supported by Significant Improvements in the Three Pillars of Clinical Management of COPD
TORCH: Conclusions
- TORCH is the first study to show a potential survival benefit of pharmacotherapy in COPD patients (with p=0.052 for SFC vs. placebo in terms of all cause mortality at 3 years)
- In addition, SFC also decreased exacerbations and improved lung function and improved and sustained quality of life over three years
- These were accompanied by a reduction in the rate of decline in lung function in patients taking SFC compared with placebo demonstrating an effect on disease progression
-
These results demonstrate the clinical efficacy of SFC 50/500 ?g bd in patients with FEV1<60% predicted.
References
1. N Engl J Med 2007; 356:775-89
2. Am J Respir Crit Care Med 2008; 178:332–338