Efficacy of Tiotropium in Patients with COPD
18 Mar, 14
Tiotropium is Effective in Indian COPD Patients
Objective
- To compare the efficacy of Tiotropium (Tiova; Cipla Ltd., India)) delivered via dry powder inhaler (DPI) versus Tiotropium (Tiova; Cipla Ltd., India) delivered via pressurized metered dose inhaler (pMDI) with a spacer in COPD patients.
Patients and Methods
- Randomized, double-blind, double-dummy, crossover study.
- 20 stable COPD patients aged 40-70 years age with smoking history of 10 pack years or non-smokers with occupational exposure.
- Subjects were included and randomized to single doses of Tiotropium (18 mcg) via DPI or via pMDI with anti-static spacer (Zerostat; Cipla Ltd., India) or placebo on three separate days, at least four days apart.
Results
1. Efficacy
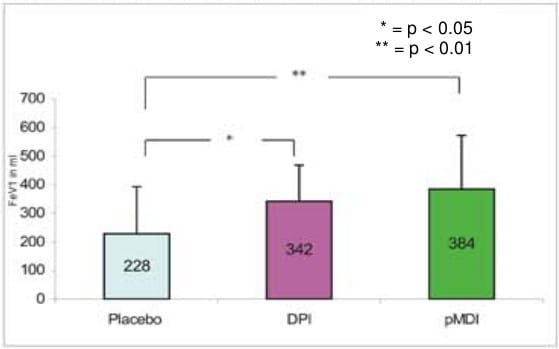
- The mean maximum change from the baseline for FEV1 was 384.1 ml with pMDI + spacer, 342.1 ml with DPI and 228 ml with placebo respectively.
Change in mean maximum FEV1 from the baseline
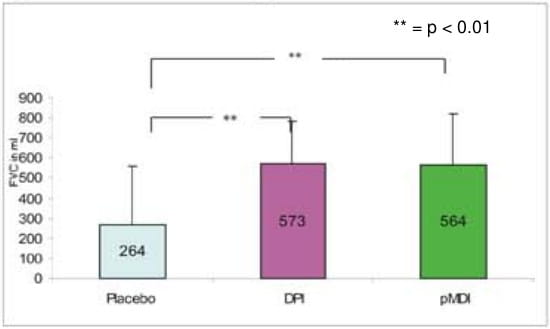
- The mean maximum change from the baseline for FVC was 564.2 ml with pMDI + spacer, 573.2 ml with DPI and 264 ml with placebo respectively.
Change in mean maximum FVC from the baseline
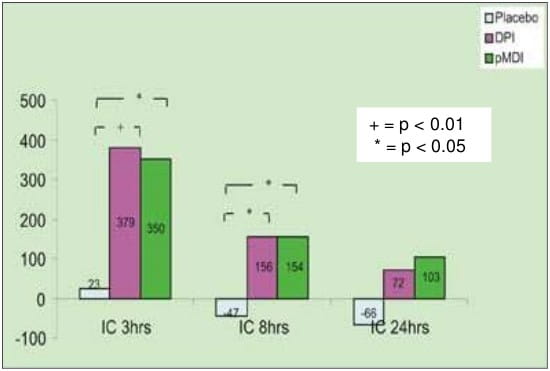
- The mean change from the baseline for IC values at different time intervals with pMDI, DPI and placebo are shown below (graph):
Conclusion
- The study recommends use of Tiotropium via pMDI with anti-static Cipla spacer in patients who prefer to use and especially in COPD patients especially those who cannot generate sufficient inspiratory flow rates.
-
Tiotropium administered via pMDI and anti-static Cipla spacer shows a superior time dependent bronchodilator response compared to placebo whereas similar efficacy to Tiotropium administered via DPI.
Respir Med 2007; 101: 2464-2471.