Safety and Efficacy of Fluticasone + Salmeterol in Patients with Asthma
18 Mar, 14
Fluticasone + Salmeterol: Role in Treatment of Bronchial Asthma
Objective
- Clinical evaluation of safety and efficacy of Fluticasone + Salmeterol (Seroflo Rotacaps; Cipla Ltd; India) given through dry powder inhaler (Rotahaler; Cipla Ltd; India) in the treatment of bronchial asthma.
Patients and Methods
- Open, multicenter, non-comparative study in clinical practice situation.
- 80 patients were enrolled by 28 investigators from 15 cities in India.
- Patients aged 12- 65 years with a diagnosis of mild to moderate asthma.
- Fluticasone + Salmeterol (100/50) Rotacaps twice daily for 4 weeks.
Results
1. Clinical Efficacy
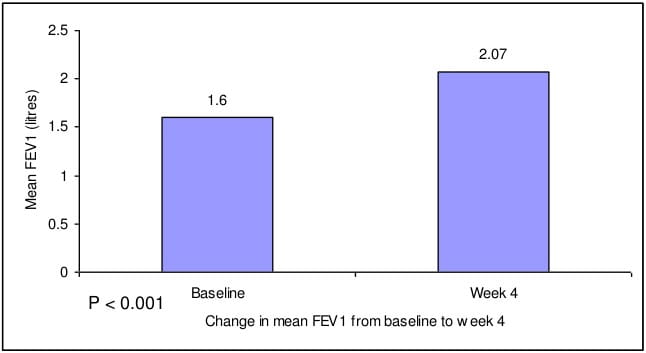
- There was significant increase (by 29.13%) in mean FEV1 from baseline after 4 weeks.
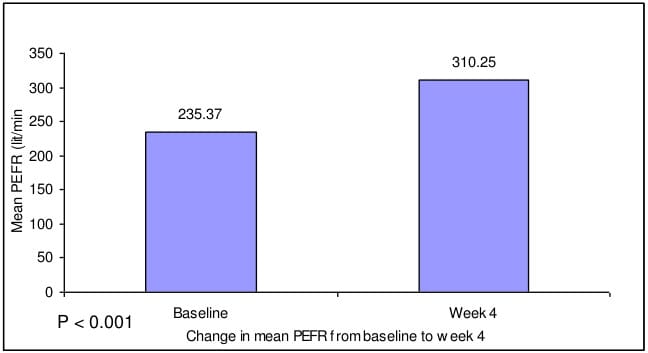
- There was significant increase (by 31.82%) in mean PEFR from baseline after 4 weeks.
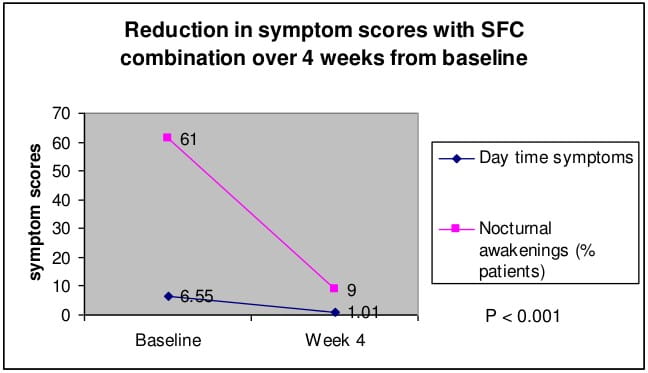
- There was significant reduction in mean daytime symptom score and mean number of nocturnal awakenings from baseline at the end of 4 weeks.
2. Safety & Tolerability
- In the present study, the fluticasone + salmeterol combination was well tolerated with the type of adverse effects reported similar to those with the two drugs.
- No patient had to discontinue therapy due to any adverse event.
- There was no increase in the incidence of adverse events.
Conclusion
- Fluticasone + Salmeterol Rotacaps given through Cipla's dry powder inhaler is an effective and well tolerated therapy in the treatment of bronchial asthma.
-
It has the potential to improve patient compliance and consequently improve asthma control.
Indian Practitioner 2001; 54: 551-557.