Safety and Efficacy of Formoterol in Patients with Asthma
18 Mar, 14
Formoterol PMDI is Safe and Effective in Indian Asthmatics
Objective
- To compare efficacy and safety of Formoterol (Foratec; 12 mcg/actuation; Cipla Ltd., India) HFA pressurized metered dose inhaler (pMDI) versus Formoterol- CFC pMDI (12 mcg/actuation; Novartis, UK).
Patients and Methods
- Randomized, double-blind, parallel-group, multicentre study.
- Subjects aged 12-65 years with confirmed diagnosis of asthma.
- 280 subjects were included and randomized to a 12 week treatment with 1 puff b.i.d. of either Formoterol-HFA pMDI or Formoterol-CFC pMDI or a placebo pMDI.
Results
1. Efficacy
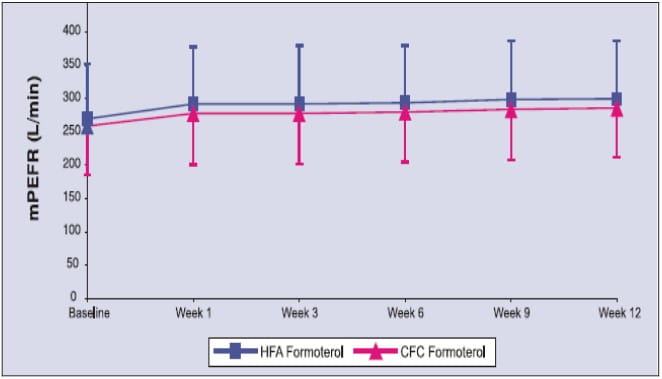
- There were no significant differences between the two treatment groups (Formoterol-HFA pMDI vs. Formoterol-CFC pMDI) in mean values of morning PEFR over 12 weeks.
Mean morning PEFR over 12 weeks
2. Safety and Tolerability
- The incidence of adverse events was similar in the two treatment groups.
- Most common adverse events were headache, rhinitis and allergic rhinitis.
Conclusion
- Formoterol-HFA pMDI is clinically as effective as Formoterol-CFC pMDI.
-
The safety profile of the two treatment groups was also similar.
P2153, presented at European Respiratory Society (ERS) Conference, 2007