Safety and Efficacy of Salmeterol in Patients with Asthma
18 Mar, 14
Salmeterol PMDI is Safe and Efective in Indian Asthmatics
Objective
- To compare efficacy and safety of Salmeterol-HFA pMDI (SEROBID; 25 mcg/actuation; Cipla Ltd., India) versus Salmeterol-CFC pMDI (25 mcg/actuation; Allen and Hanburys, UK).
Patients and Methods
- Randomized, double-blind, parallel-group, multicentre study.
- Subjects aged 12-65 years with confirmed diagnosis of asthma.
- 277 subjects were included and randomised to a 12-week treatment with 2 puffs b.i.d. of either Salmeterol-HFA pMDI or Salmeterol-CFC pMDI or a placebo pMDI.
Results
1. Efficacy
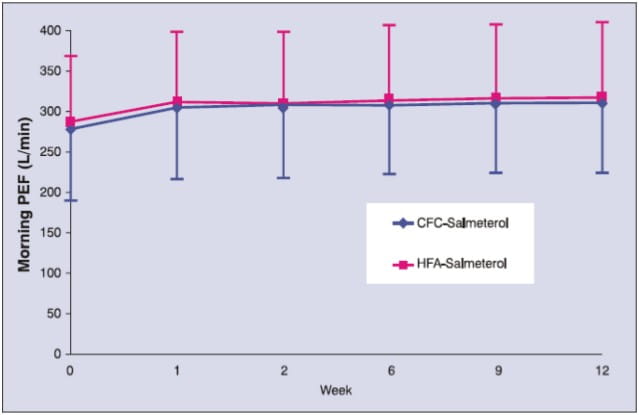
- There were no significant differences between the two treatment groups (Salmeterol-HFA pMDI vs. Salmeterol-CFC pMDI) in mean values of morning PEF over 12 weeks.
Morning PEF (L/min) over 12 weeks
2. Safety and Tolerability
- Adverse events of similar nature and number were reported in both treatment groups.
Conclusion
- Salmeterol-HFA pMDI is clinically as effective as salmeterol-CFC pMDI.
-
The safety profile of the two treatment groups was also similar.
P2151, presented at European Respiratory Society (ERS) Conference, 2007