Clarithromycin: An Emerging Treatment Recourse in Lower Respiratory Tract Infections
Introduction
Out of a total of 3,941,000 deaths in the world, respiratory tract infection accounts for 34.60% deaths in the Southeast region of the world. The aetiology and symptomatology of respiratory tract diseases varies based on age, gender, season, type of population at risk and other factors. Lower respiratory tract infections (LRTIs) are one of the most common infectious diseases in humans worldwide and pose a huge burden on the society. About 4.4% of all hospital admissions and 6% of all general practitioner consultations in the world are mainly due to LRTIs. The mortality and morbidity is comparatively high in all age groups.1
The term, LRTI, covers a wide spectrum of diseases, including acute bronchitis, acute exacerbations of chronic bronchitis (AECB) and community-acquired pneumonia (CAP).2
A variety of organisms are usually implicated in the aetiology of LRTI. These causative agents vary from area to area and also vary in their antibiotic susceptibility profile. Gram-positive bacteria such as Staphylococcus aureus, Streptococcus pneumoniae, etc., and Gram-negative bacteria such as Haemophilus influenzae, Pseudomonas spp., Acinetobacter spp., and Klebsiella spp. have been recovered from LRTIs. The antimicrobial therapy for LRTIs is frequently empirical and presumptive.1
Role of Macrolides in Community-Acquired Pneumonia
In many cases with community-acquired pneumonia (CAP), the most common pathogens responsible for hospitalization are Streptococcus pneumoniae, Haemophilus influenzae, Legionella pneumophila and Mycoplasma pneumoniae.3
The macrolides are particularly useful because of their spectrum of activity that covers most of the respiratory pathogens encountered in hospitalized patients as well as in patients who can be treated at home. Their intracellular concentrations and their concentrations in the bronchial mucosa, in lung biopsy specimen, alveolar macrophages, sputum and in the alveolar lining fluid make them excellent antibiotics for the treatment of such infections both in the community and in the hospital.3 If Legionella, Mycoplasma or Chlamydia spp., so-called 'atypical' pathogens, are involved, macrolide antibiotics are the therapy of first choice.4
Prognostic Scoring Systems for CAP
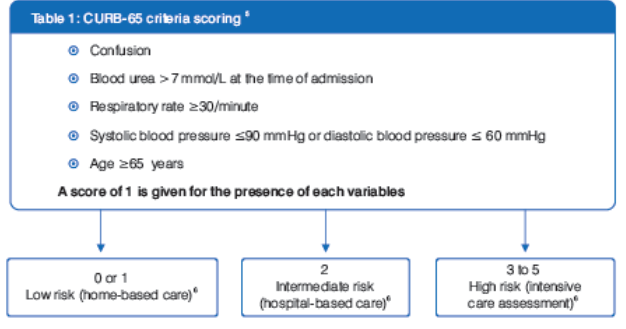
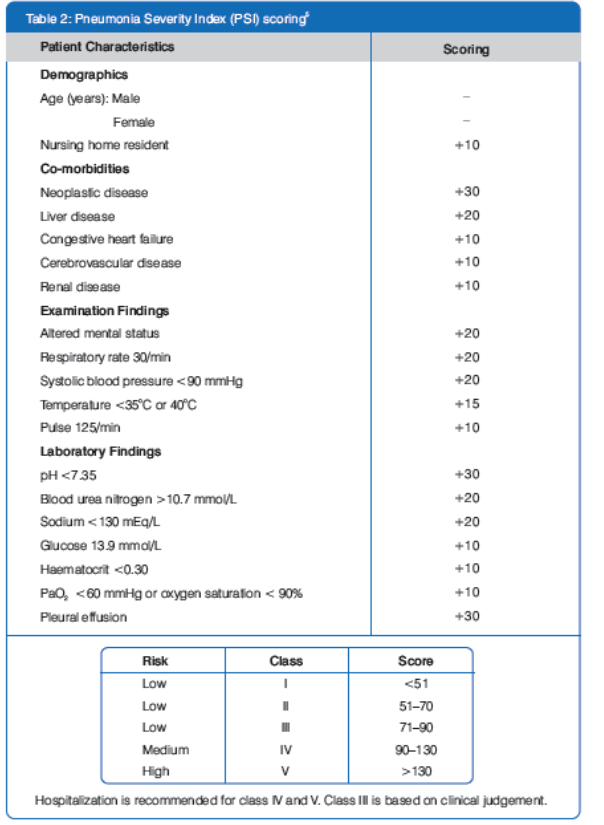
The knowledge of relevant prognostic factors might be useful for early identification of CAP patients at high risk requiring intensive care treatment. Two prognostic scoring systems have been developed to address these issues, as shown in Table 1 and Table 2.5
Mortality rates progressively increase with increasing risk class in both severity scoring systems.5
Parenteral Therapy
Parenteral antibiotic therapy is essential as initial therapy for critically ill patients with severe pneumonia or bacteraemic pneumococcal pneumonia and for patients who are unable to take oral medications.7 Intravenous (I.V.) antibiotics rapidly reach bactericidal levels in the serum and cross the alveolar-capillary membrane to reach alveolar lining fluid and interstitium. With I.V. antibiotic therapy, accumulation of antibiotic in the lung is rapid.8 Patients treated initially with parenteral antibiotics should be transferred to an oral regimen as soon as clinical improvement occurs and the temperature has been normal for 24 hours, provided there is no contraindication to the oral route.9
Current Treatment Guidelines for CAP
Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) Guidelines for CAP in Adults-200710
Inpatient, Non-ICU (Intensive Care Unit) Treatment
- A ?-lactam plus a macrolide (Strong recommendation; Level I evidence).*10
- Patients should be switched from I.V. to oral therapy when they are haemodynamically stable and improving clinically, are able to ingest medications, and have a normally functioning gastrointestinal tract (Strong recommendation; Level II evidence).*10
*IDSA Guidelines: Level I: High; Evidence from well-conducted, randomized controlled trials. | Level II: Moderate; Evidence from well-designed, controlled trials without randomization (including cohort, patient series, and case-control studies). Level II studies also include any large case series in which systematic analysis of disease patterns and/or microbial aetiology was conducted, as well as reports of data on new therapies that were not collected in a randomized fashion.
Joint Indian Chest Society (ICS) and National College of Chest Physicians (NCCP) Recommendations for CAP-201211
Antibiotic Therapy in the Hospitalized Non-ICU Setting
- The recommended regimen is a combination of a -lactam plus a macrolide (preferred ? -lactams include cefotaxime, ceftriaxone and amoxycillin-clavulanic acid) (Grade A; Level I evidence).11
Antibiotic Therapy in ICU Setting
The recommended regimen is a ? -lactam (cefotaxime, ceftriaxone or amoxycillin-clavulanic acid) plus a macrolide for patients without risk factors for Pseudomonas aeruginosa (Grade A; Level II evidence. 11
ICS/NCCP Recommendations: Level I: High-quality evidence backed by consistent results from well-performed randomized controlled trials or overwhelming evidence from well-executed observational studies with strong effects. | Level II: Moderate-quality evidence from randomized trials (that suffer from flaws in conduct, inconsistency, indirectness, imprecise estimates, reporting bias or other limitations). | Grade A: Strong recommendation to do (or not do) where the benefits clearly outweigh the risk (or vice versa) for most, if not all patients.
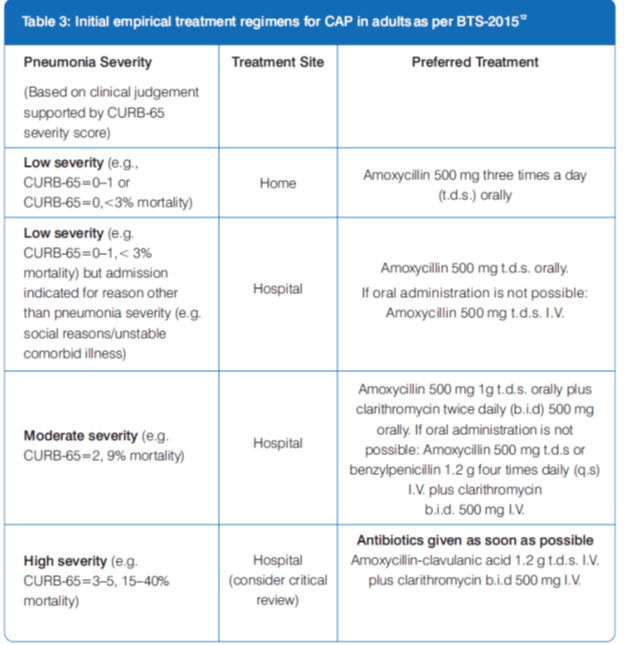
British Thoracic Society (BTS) Guidelines for the Management of CAP-2009, annotated 201512
Clarithromycin I.V. in the Treatment of CAP
Clarithromycin, a macrolide antibiotic, is highly effective, achieves acceptable serum levels and reaches concentrations in pulmonary tissue that exceed the minimum inhibitory concentrations (MICs) for atypical and typical pathogens, including most macrolide-resistant Streptococcus pneumonia. 13
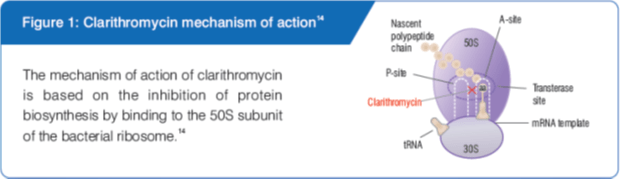
Mode of Action
Other Beneficial Actions of Clarithromycin
- Macrolides exert local immunomodulatory effects and have anti-inflammatory properties. Clarithromycin has shown in vitro suppression of cytokines and can modulate cytokine production in vivo in humans with CAP.15,16
- Macrolides have been shown to inhibit biofilm formation and decrease mucus hypersecretion, leading to improved mucociliary clearance.17
- Additionally, clarithromycin has a post-antibiotic effect.18
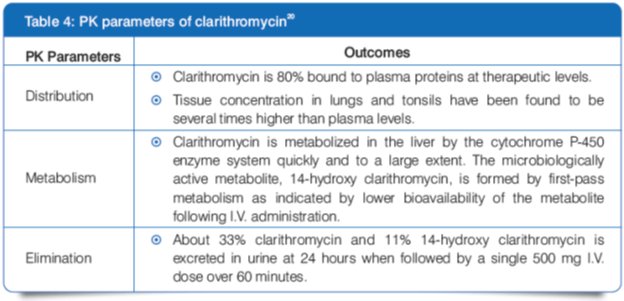
Pharmacokinetics (PK)
- Steady state is achieved by 3 days of IV dosing. 20
- In patients with renal impairment, an increase in clarithromycin plasma levels and its active metabolite can be observed.20
Pharmacodynamics
Spectrum
Clarithromycin is a macrolide antibiotic whose spectrum of activity includes many Gram-positive organisms such as Staphylococcus aureus, Streptococcus pneumoniae and Streptococcus pyogenes; Gram-negative aerobic bacteria such as Haemophilus influenzae, Haemophilus parainfluenzae and Moraxella catarrhalis; and Atypical aerobic organisms, including Legionella pneumophila, Mycoplasma pneumoniae and Chlamydophila pneumoniae. It also has activity against many anaerobic bacteria, some mycobacteria and other organisms, including Ureaplasma, Chlamydia, Toxoplasma and Borrelia. Clarithromycin is usually bacteriostatic, but may be bactericidal depending on the organism and drug concentration.13,21
Clarithromycin has demonstrated bactericidal activity against both typical and atypical respiratory pathogens. 22
Aerobic Gram-negative micro-organisms such as Escherichia coli, Klebsiella spp. and Pseudomonas aeruginosa are inherently resistant to clarithromycin.20
Clinical Efficacy and Safety Evidence
Clarithromycin – an Ideal Antibiotic for the Treatment of CAP23
Study Design
A total of 290 CAP patients were treated with clarithromycin from January 1992 to December 1997, out of which
- 163 were males (98 smokers) and 127 were females (41 smokers);
- 87 were over 65 years old and 203 had concomitant diseases (mainly cardiovascular); and,
- 172 patients were admitted after unsuccessful therapy (122 cephalosporins and 48 penicillins).
Study Dose
Clarithromycin I.V. 500 mg was first given b.i.d. in 250 or 500 mL of saline solution and then switched after 4–5 days to the same dosage given orally.
Results
Conclusions
Clarithromycin showed good antimicrobial spectrum, PK profile and the possibility of I.V. to oral sequential administration. Hence, it can be used as an ideal antibiotic in the treatment of CAP.
Combination of ?-lactam and Clarithromycin in the Treatment of Moderately Severe CAP Offers Early Clinical Stability24
Study Design
An open-label, multicentre, non-inferiority, randomized trial (n=580) in immunocompetent adult patients hospitalized for moderately severe CAP. Mean PSI score was 84.
Study Dose
Patients were randomized into two groups:
- 291 patients in the monotherapy arm received initial treatment with ?-lactam alone (I.V. cefuroxime 1.5 g t.d.s or I.V. amoxycillin and clavulanic acid 1.2 g q.d.s).
- 289 patients in the combination arm received a ?-lactam and a macrolide (clarithromycin 500 mg BD intravenously or orally).
Urine samples were systematically tested for the presence of Legionella pneumophila (an atypical pathogen) antigen, and a macrolide was added to the monotherapy arm in case of a positive test result. Median time before administration of clarithromycin was 47 hours in patients with Legionella pneumophila infection in the monotherapy arm. Streptococcus pneumoniae was the most common pathogen.
Results
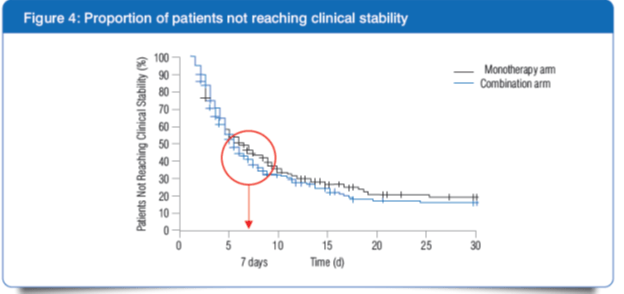
1] Higher number of patients did not reach clinical stability in the monotherapy arm as compared with the combination arm (41.2% vs 33.6%, P = 0.07) after 7 days of treatment.
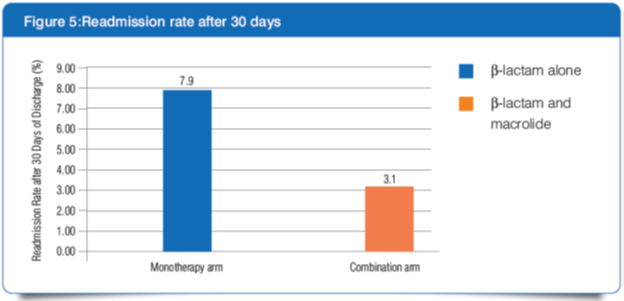
2] At 30 days after discharge, more patients in the monotherapy arm had been re-admitted than those in the combination arm (7.9% vs. 3.1%, P = 0.01).
Conclusion
- Initial empirical treatment with ?-lactam monotherapy delays the clinical stability for patients infected with atypical pathogens. The superiority of combination therapy may be attributed to the timely coverage of Legionella infection.
- Patients with higher severity of pneumonia (PSI category IV or CURB-65 score of ≥2) seemed also to benefit from combination therapy.
- Better control of the host inflammatory response through a non-antibiotic effect of clarithromycin might have protected patients in the combination arm from adverse events that led to readmission.
Advantages of Clarithromycin over Azithromycin
- Clarithromycin is more active when compared with azithromycin in terms of MIC values.25
- Although both drugs are highly active against typical and atypical pathogens, several studies have found the MICs of clarithromycin to be 1 to 2 dilutions lower than the MICs of azithromycin against pathogens commonly encountered in CAP.13
- Clarithromycin seems to be more active than azithromycin against atypical pathogens such as Legionella pneumophila and Chlamydia pneumoniae.26
- The levels of clarithromycin achieved in the epithelial lining fluid and alveolar macrophages are higher than those of azithromycin.27
- Azithromycin attains lower serum concentrations than clarithromycin. So, it should not be used in patients suspected of having bacteraemic pneumonia.26
- The lower mutant prevention concentration (MPC) values for clarithromycin (MPC90=0.5 mg/L) vs azithromycin (MPC90=4 mg/L) reduces the likelihood for resistance selection from susceptible populations.25
- Azithromycin was found to be generally somewhat less active than clarithromycin in terms of inhibition of the production of total (intracellular+extracellular) pneumolysin, a pneumococcal toxin, by the macrolide susceptible- and two macrolide-resistant strains of Streptococcus pneumoniae.28
- In the time-kill experiments against Streptococcus pneumoniae, clarithromycin showed significantly greater killing effects at 1 hour than did azithromycin. Also, azithromycin had a significantly lower mean post-antibiotic effect than clarithromycin in clinical isolates of Streptococcus pneumoniae. 29
Clarithromycin I.V.: Place in Therapy for CAP
Several retrospective studies suggest superiority of beta lactam-macrolide combination therapy in hospitalized CAP patients, particularly those with more severe CAP. As a rule of thumb, the more severely the patient presents, the stronger is the recommendation for such combination treatment.30
Clarithromycin has an established efficacy, safety and tolerability in the treatment of CAP.13,23 As per the recommendations by the current guidelines, I.V. clarithromycin can be added to a ?-lactam agent (e.g. amoxycillin/clavulanic acid) in the empirical treatment of moderate-to-severe CAP in patients who require hospital admission in non-ICU as well as ICU settings.9,10,11
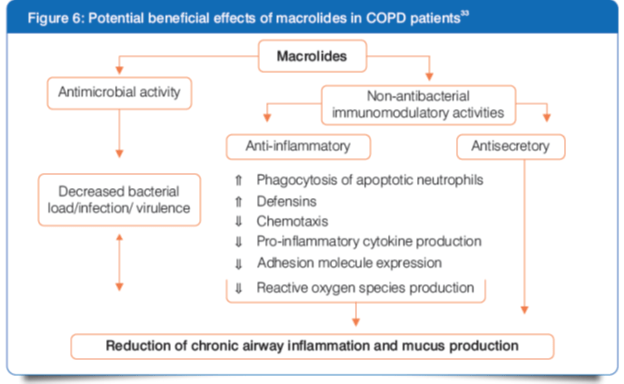
Role of Macrolides in Acute Exacerbations of Chronic Bronchitis/Acute Exacerbation of Chronic Obstructive Pulmonary Disease
- Approximately 5–10% of the adult world population is suffering from chronic obstructive pulmonary disease (COPD).31
- The progressive course of COPD is accelerated by acute exacerbations (AECOPD), which are episodes of worsening symptoms, and are the most frequent causes of hospitalizations and death among COPD patients.32
- Bacterial infection is likely aetiologic in approximately 50% of AECOPD patients.33
- The pre-eminent pathogens associated with acute exacerbation of chronic bronchitis (AECB) are Haemophilus influenzae, Streptococcus pneumoniae and Moraxella catarrhalis, which together account for 85–95% of bacterial exacerbations.34
- As per the guidelines, AECB (or AECOPD) should be treated with oral or, occasionally, I.V. antibiotics if repeated episodes of cough and dyspnoea occur and if there is evidence for increased sputum production, and if this sputum is purulent.3,35
- Macrolide antibiotics provide adequate coverage for the most frequently identified pathogens in AECB/AECOPD.33
- Macrolide antibiotics have assumed an important role in the management of AECOPDs because of their broad-spectrum coverage and excellent safety profile.33
Clarithromycin in the Treatment of AECB/AECOPD
The effects of clarithromycin on the inhibition of inflammatory cytokine production by COPD sputum cells is more potent than that of azithromycin.31
Clarithromycin I.V.: Place in Therapy for AECB/AECOPD
I.V. clarithromycin might be used in hospitalized patients with severe COPD exacerbations who have clinical signs of a bacterial infection, according to the guidelines for COPD.30,35
Take-Home Message
- CAP and AECB or AECOPD are the leading causes of mortality and morbidity that require immediate treatment with empirical therapy.
- Macrolides are considered as a part of primary treatment in moderate-to-severe CAP and also as an additional treatment option for severe AECB/AECOPD due to their safety and efficacy.
- Clarithromycin I.V. can be recommended for wide application due to the following properties:
- Safety and efficacy
- Wide antimicrobial spectrum and superior PK profile
- Immunomodulatory and anti-inflammatory properties
- High serum concentration and better penetration into pulmonary tissues
- Post-antibiotic effect
References
1. Purti CT and Kiran D. Lower Respiratory Tract Infections: Current etiological trends and antibiogram. J Pharm Biomed Sci. 2014;04(03):249–255.
2. Treatment of lower respiratory tract infections. Available from: http://www.stjames.ie/GPsHealthcareProfessionals/Newsletters/NMICBulletins/NMICBulletins1996/VOL2-2.pdf. Accessed on September 20, 2017.
3. Rubinstein E and Levy I. Macrolides as First Line Therapy in Adult Lower Respiratory Tract Infections: Pros and cons. Clin Microbiol Infect. 1996;1(2):S23–S26.
4. Vogel F. A guide to the treatment of lower respiratory tract infections. Drugs. 1995;50(1):62–72.
5. Shah BA, Ahmed W, Dhobi GN, et al. Validity of pneumonia severity index and CURB-65 severity scoring systems in community acquired pneumonia in an Indian setting. Indian J Chest Dis Allied Sci. 2010;52(1):9–17.
6.Pneumonia in Adults: Diagnosis and management. Available from: https://www.nice.org.uk/guidance/cg191/resources/pneumonia-in-adults-diagnosis-and-management-35109868127173. Accessed on August 17, 2017.
7.Cunha BA. Oral or intravenous-to-oral antibiotic switch therapy for treating patients with community acquired pneumonia. Am J Med. 2001;111(5):412–413.
8.Siegel RE, Halpern NA, Almenoff PL, et al. A Prospective Randomized Study of Inpatient Intravenous Antibiotics for Community-acquired Pneumonia: The optimal duration of therapy. Chest. 1996;110:965–971.
9.Summary of Recommendation: BTS Guideline for the management of CAP 2009, annotated 2015. Available from: https://www.brit-thoracic.org.uk/guidelines-and-quality-standards/community-acquired-pneumonia-in-adults- guideline/annotated-bts-guideline-for-the-management-of-cap-in-adults-2014/. Accessed on August 18, 2017.
10.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(2):S27–72.
11. Gupta D, Agarwal R, Aggarwal AN, et al. Guidelines for Diagnosis and Management of Community-and Hospital-acquired Pneumonia in Adults: Joint ICS/NCCP(I) recommendations. Lung India. 2012;29(2):S27–62.
12.2015 - Annotated BTS guideline for the management of CAP in adults (2009) Summary of recommendations. Available from: https://www.brit-thoracic.org.uk/document-library/clinical-information/pneumonia/adult-pneumonia/annotated-bts- cap-guideline-summary-of-recommendations/. Accessed on August 17, 2017.
13.McCarty JM. Clarithromycin in the management of community-acquired pneumonia. Clin Ther. 2000;22:281–294.
14.Available from : http://www.antibiotics-info.org/clarithromycin.html. Accessed on August 17, 2017.
15.Kovaleva A, Remmelts H, Rijkers GT, et al. Immunomodulatory Effects of Macrolides during Community-acquired Pneumonia: A literature review. J Antimicrobial Chemother. 2012;67(3):530–540.
16.Amsden GW. Anti-inflammatory effects of macrolides—An underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J Antimicrobial Chemother. 2005;55(1):10–21.
17. Sligl WI, Asadi L, Eurich DT, et al. Macrolides and Mortality in Critically Ill Patients with Community-acquired Pneumonia: A systematic review and meta-analysis. Crit Care Med. 2014;42(2):420–432.
18. Rodvold KA. Clinical Pharmacokinetics of Clarithromycin. Clin Pharmacokinetics. 1999;37(5):385-398.
19. Gaynor M and Mankin AS. Macrolide Antibiotics: Binding site, mechanism of action, resistance. Curr Top Med Chem. 2003;3(4):949–961.
20. Cipla-Synclar (Lyophilized) Prescribing Information Leaflet.
21.Clarithromycin. Available from: https://www.drugbank.ca/drugs/DB01211. Accessed on September 20, 2017.
22.LeBel M. Pharmacokinetic Properties of Clarithromycin: A comparison with erythromycin and azithromycin. Can J Infect Dis. 1993;4(3):148–152.
23.Parola D, Dell'Orso D and Terzano C. Efficacy and safety of clarithromycin in the treatment of community-acquired pneumonia. Recenti Prog Med. 2000;91(1):12–15.
24.Garin N, Genn? D, Carballo S, et al. ?-Lactam Monotherapy vs ?-lactam-macrolide Combination Treatment in Moderately Severe Community-acquired Pneumonia: A randomized noninferiority trial. JAMA Intern Med. 2014;174(12):1894–1901.
25.Blondeau J, Chan CK and Davidson R. Judicious Use of Antibiotics to Minimize Emerging Resistance: The macrolide clarithromycin as a case study. Clin Pract. 2013;10(3):359–370.
26.Zuckerman JM. Macrolides and Ketolides: Azithromycin, clarithromycin, telithromycin. Infect Dis Clin North Am. 2004;18(3):621–649.
27.Blondeau JM. Update on the use of the macrolides for community-acquired respiratory tract infections. Therapy. 2006;3(5):619–650.
28.Anderson R, Steel H, Cockeran, et al. Comparison of the effects of macrolides, amoxicillin, ceftriaxone, doxycycline, tobramycin and fluoroquinolones, on the production of pneumolysin by Streptococcus pneumoniae in vitro. J Antimicrob Chemother. 2007;60(5):1155–1158.
29.Fuursted K, Knudsen JD, et al. Comparative Study of Bactericidal Activities, Postantibiotic Effects, and Effects on Bacterial Virulence of Penicillin G and Six Macrolides against Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41(4):781–784.
30. Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections - Full version. Clin Microbiol Infect. 2011;17(6):E1–E59.
31.Ni W, Shao X, Cai X, et al. Prophylactic Use of Macrolide Antibiotics for the Prevention of Chronic Obstructive Pulmonary Disease Exacerbation: A meta-analysis. PLoS One. 2015;10(3):e0121257.
32.Wilson R, Sethi S, Anzueto A, et al. Antibiotics for treatment and prevention of exacerbations of chronic obstructive pulmonary disease. J Infect. 2013;67(6):497–515.
33.Martinez FJ, Curtis JL and Albert R. Role of macrolide therapy in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3(3):331–350.
34.Ball P. Epidemiology and treatment of chronic bronchitis and its exacerbations. Chest. 1995;108(2):43S–52S.
35.Vestbo J, Hurd SS, Agusti AG, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365.