COPD Management Beyond the Lungs:Heart Failure
COPD: Not Just a Lung Disease
Today, emerging evidence on the systemic effects of COPD (chronic obstructive pulmonary disease) implies that it is much more than just a respiratory disease. Hence, it becomes imperative for a practicing clinician to reflect not just on the lungs but also the human body as a whole.1
The CIRO CO morbidity (CIROCO) study presented at the American Thoracic Society (ATS) Conference, 2012, revealed that approximately 50% of COPD patients had five or more comorbidities. Cardiac conditions were amongst the most frequent comorbidities.2
COPD has been linked with many concomitant cardiac conditions such as CHF (chronic heart failure) and coronary artery disease.3 This issue of COPD: Beyond the ‘lungs’ dwells on heart failure, with special emphasis on the use of beta blockers in patients with COPD and heart failure.
Heart Failure and COPD
Burden
The prevalence of CHF in COPD patients is recognized to range from 20% to 32%.4
COPD patients are 4.5 times more likely to develop heart failure as compared with control individuals without COPD, independent of age and other cardiovascular risk factors.5
Around 25% of patients with COPD aged 65 years or over have unrecognized CHF.6The rate-adjusted hospital prevalence of CHF is 3 times more among patients discharged with a diagnosis of COPD in contrast to patients discharged without a diagnosis of COPD.7
Why They Often Occur Together
Smoking
Smoking has been implicated as an important risk factor for both COPD and heart failure.4 Conventionally, smoking has been linked to COPD, whereas it is also the second most important and independent risk factor for the development of CHF.8 After adjusting for other known risk factors for CHF, cigarette smoking has been found to increase the risk of CHF in men by 45% and in women by 88%.9
Chronic systemic inflammation
Chronic systemic inflammation has also been postulated as a cause for the coexistence of COPD and CHF in patients.4 In patients with COPD and concomitant heart failure, high levels of circulatory pro-inflammatory cytokines have been reported.10 Low-grade systemic inflammation may hasten the progression of coronary atherosclerosis and ultimately lead to ischaemic cardiac myopathy.4
Over-activation of the common pathways in COPD and CHF, causing mutual disease progression
There are common pathways in COPD and CHF that cause mutual disease progression, such as:
- over-activation of the renin-angiotensin system (RAS); and,
- over-activation of the sympathetic nervous system, causing systemic and direct cardiac effects.6
Metabolic modulation
In the advanced stages of both diseases, metabolic modulation has been reported at the cellular level, namely a shift from glucose metabolism to lipid metabolism. As a consequence, there are generalized skeletal muscle alterations, which cause a decrease in muscle mass, size and diameter and, eventually, result in chronic wasting and cachexia in the end stage of both COPD and heart failure.6, 10
Poor lung function linked with heart failure
Poor lung function has also been linked with heart failure in the 2012 Atherosclerosis Risk in Communities (ARIC) study. This was a large population based cohort study (n=13,660 participants) with long-term follow-up, and reported low forced expiratory volume in one second (FEV ) and an obstructive airway disease as strong and independent predictors of the risk of incident heart failure.11
Diagnosis
In clinical practice, diagnostic assessment of heart failure in the presence of COPD is tricky considering the significant overlap in symptoms and signs such as:
- breathlessness,
- fatigue, and
- chest pain on exertion12-14
Due to this sign-symptom overlap, up to 80% of concomitant heart failure in elderly patients with COPD remains unrecognized in day-to-day practice, particularly in the early stages of the disease.15
Diagnostic tests such as chest X-ray, electrocardiography (ECG) and spirometry are reported to be less sensitive in heart failure patients with comorbid COPD.12
Table 1: Factors that make the differential diagnosis of heart failure and hypoxaemic COPD challenging10, 16
|
Investigations |
Problem |
|
History |
|
|
Physical examination |
|
|
ECG |
|
|
Chest X-rays |
|
|
Echocardiography |
|
It has been suggested that in the sub-group of COPD patients with heart failure, evaluation of serum levels of natriuretic peptides (BNP or NT-proBNP) levels may help.6 The negative predictive value may be most useful as natriuretic peptides work better in excluding, rather than for diagnosing, heart failure10 (Table 2).
Table 2: Diagnostic utility of serum natriuretic peptides in excluding CHF in COPD
|
Setting |
Serum levels of natriuretic peptides that make heart failure unlikely6 |
|
|
In acute setting |
BNP less than 100 pg/ml |
NT-proBNP less than 300 pg/ml |
|
In non-acute setting |
BNP less than 35 pg/ml |
NT-proBNP less than 125 pg/ml |
Echocardiography is the cornerstone for establishing a diagnosis of heart failure.6
Practice Pointer
In patients with COPD, echocardiography and measurement of serum natriuretic peptides should be performed to look for early heart failure.6
Impact
Community-dwelling elderly COPD patients with newly detected heart failure are reported to have twice the mortality rate compared with those without heart failure (25.6% versus 12.1%, p=0.002 during mean ± SD: 4.2 ± 1.4 years follow-up), also independent of other factors.17
In patients hospitalized with a first episode of heart failure, the presence of COPD has been linked with a significantly lower 5-year survival rate compared with those without COPD (31% versus 42%, p=0.03).18
Patients with COPD or CHF have skeletal muscle changes that restrict the functional capacity independently of primary organ failure. The additive effects of both COPD and CHF on skeletal muscle may further worsen the functional capacity and escalate the chances of clinical deterioration and hospitalization.19
Treatment modalities
Pharmacological therapy for heart failure includes:
- diuretics;
- angiotensin-converting enzyme (ACE) inhibitors;
- beta-blockers;
- angiotensin-receptor blockers;
- mineralocorticoid-receptor antagonists;
- digoxin; and,20
- ivabradine
The treatment of heart failure in COPD is primarily the same as in those without COPD, including cardio-selective beta-blockers.6 However; controversy surrounds the use of beta-blockers in COPD patients.21
This issue of COPD: Beyond the Lungs examines the role of beta-blockers in patients with COPD and concomitant CHF.
Overview of Beta-Blockers
The Nobel Committee's comment on the discovery of propranolol in 1988: “The greatest breakthrough when it comes to pharmaceuticals against heart illness since the discovery of digitalis 200 years ago”.22
Post-discovery, beta-blockers have definitely brought a transformation in the practice of medicine.22
Mode of action
Symptomatic heart failure leads to activation of neurohormonal mechanisms, including the sympathetic nervous system and the RAS system. Chronic neurohormonal activation increases the heart rate and cardiac output and, as a consequence, leads to an increased myocardial oxygen demand, ischaemia and oxidative stress.23
The increase in sympathetic activity may deleteriously affect the survival. Several studies have reported that blockade of beta-adrenergic receptors improves symptoms and enhances survival in patients with heart failure.23
Based on the published evidence of several trials demonstrating the efficacy and the survival benefits of beta-blockers, these agents are considered the cornerstone of systolic heart failure management. This marks an important shift, given that betablockers were previously contraindicated in these patients because of their negative inotropic action.23
Various mechanisms are postulated to contribute to the beneficial effect of betablockers in heart failure:
- Decrease in circulating levels of vasoconstrictors
- Decrease in blood pressure and heart rate
- Up-regulation of myocardial beta -receptor density, thus enhancing the contractile function1
- Decrease in myocardial gene production of inflammatory cytokines
- Positive effects on cardiac remodelling
- Decrease in myocardial oxygen consumption and increase in diastolic perfusion
- Normalization of the expression of numerous myocardial genes that are implicated in the development of pathologic hypertrophy23
Types of beta-blockers
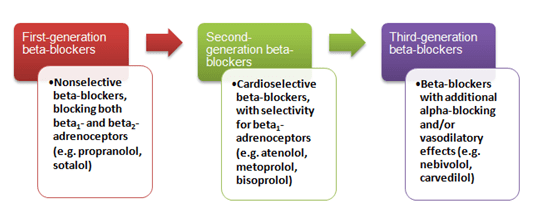
Beta-blockers may be divided into first-, second- and third-generation agents(Figure 1).24
Figure1: Beta-blockers along with their pharmacological characteristics
Beta1-selectivity is of major clinical interest because at clinically used doses, beta1-selective agents block cardiac beta-receptors with minor effects on bronchial and vascular beta-receptors (positioning of beta-receptors listed in Table 3).25
Table 3: Positioning of beta-receptors4,26
|
Beta-adrenoceptors in human airways |
Beta-adrenoceptors in human heart |
|
Beta1:10% in submucosal glands, 30% on alveolar walls Beta2:Predominanton bronchial smooth muscle |
Beta1/Beta2in ventricles:70-80%/30-20% Beta1/Beta2in atria:60-70%/40-30% |
Beta-Blockers in Concomitant Heart Failure and COPD: An Often-Missed Opportunity!
In routine practice, despite the anticipated benefits, clinicians have been reluctant to prescribe beta-blockers in patients with COPD.3 For instance; the Euro Heart Failure Survey showed that in patients with CHF, COPD was found to reduce the possibility of receiving a beta-blocker by 65%.27
Conventionally, these agents have been contraindicated in COPD, chiefly based on anecdotal evidences and case reports mentioning acute bronchospasm post- administration.4
The diminished use of beta-blockers in patients with COPD is a matter of worry since many patients with COPD ultimately die of cardiovascular causes and from ischaemic heart disease in particular.28
Use of Beta-Blockers in COPD: Key Studies to Know
- Beta-Blockers may reduce mortality and risk of exacerbations in patients with COPD29
An observational cohort study assessed data from the electronic medical records of 2,230 patients with incident or prevalent COPD between the time period from 1996 and 2006. Of the patients, 45% had cardiovascular comorbidities. Not all received beta-blockers (only 29.8%), but more than 80% of those used were cardioselective.
Key findings
- During the follow-up, it was observed that the use of beta-blockers significantly decreased the percentage of patients with at least one exacerbation.
- Beta-blockers also reduced the mortality over the follow-up period.
Figure 2: Exacerbations and mortality in patients using/not using beta-blocker therapy
- Cardio-selective beta-blockers do not produce adverse respiratory effects and, hence, should not be withheld from patients with COPD30
This review evaluated the effect of cardio-selective beta-blockers on the respiratory function of patients with COPD, using data from 22 randomized, placebo-controlled trials. A beta1-blocker was administered as a single dose or continued treatment (ranging from 2 days to 16 weeks), and the response to an inhaled or intravenous beta2-agonist after the beta1-blocker administration was measured.
Key findings
- Cardio-selective beta-blockers did not produce any significant change in FEV1 or respiratory symptoms compared with placebo.
- They did not affect the FEV1 response to the inhaled beta2-agonist.
- Results did not differ significantly in patients with comorbid cardiovascular conditions such as hypertension, angina and heart failure.
In addition, various other studies have shown the following:
- Beta-blocker therapy reduces the respiratory-related hospital admissions and emergency oral steroid prescriptions, and does not worsen lung function (88% of beta-blockers were cardio-selective).31
- Cardio-selective beta-blockers are independently associated with reduced 30-day mortality in patients with COPD undergoing vascular surgery:
- Intensified dose of cardio-selective beta-blocker (dose ≥25% of the maximum recommended therapeutic dose) is associated with both reduced 30-day and long-term mortality in patients with COPD.
- Low dose of cardio-selective beta-blockers (dose <25% of the maximum recommended therapeutic dose) leads to reduction only in long-term mortality.32
- Beta-blocker therapy does not deleteriously impact the health-related quality of life in patients with peripheral arterial disease and COPD (more than 80% were cardio-selective).33
- Third-generation cardio-selective beta-blocker therapy does not affect airway patency in hypertensive patients with COPD.34
- The use of carvedilol (a third-generation non-selective beta-blocker) is associated with lower 60- to 90-day mortality risk in patients with COPD and heart failure.35
- Although cardiovascular benefits are similar, bisoprolol has more favourable effects on lung function and less pulmonary adverse events compared with carvedilol in patients with COPD and heart failure.36
- In patients with heart failure, continuing cardio-selective beta-blockers during hospitalization for an exacerbation of COPD appears to be safe.37
Using Beta-Blockers in COPD: Answering Some Clinical Questions
Note: There is limited guidance on the use of beta-blockers in COPD patients with heart failure; some key pointers for the safe initiation of therapy in COPD patients with heart failure have been published.
Which Beta-Blockers Should Be Used In COPD And Concomitant Heart Failure?
- Cardio-selective beta-blockers may be the preferable option; metoprolol, bisoprolol and nebivolol (indicated in elderly patients with heart failure) are potential candidates.20
- The use of non-selective beta-blockers is not recommended because of the increased risk of bronchospasm. However, there is some evidence that carvedilol (non-selective with alpha-blocking activity) can be used safely.4
What Dosing Should Be Used?
- In COPD, it is recommended to initiate beta-blockers at a low dose and then gradually up-titrate (the 'start low, go slow' approach).38
- During the up-titration of beta-blockers, it is important to monitor the patient's pulse rate, blood pressure and the clinical status, to avoid side-effects such as symptomatic bradycardia and symptomatic hypotension.39
- Table 4 lists the initiating and maximum recommended doses of common drugs.
Table 4: Dosing of common beta-blockers used in heart failure20,40
|
Agent |
Initiating dose |
Maximum recommended dose |
|
Extended-release metoprolol succinate |
12.5 or 25 mg once daily |
200 mg once daily |
|
Bisoprolol |
1.25 mg once daily |
10 mg once daily |
|
Nebivolol |
1.25 mg once daily |
10 mg once daily |
|
Carvedilol |
3.125 mg twice daily |
25 to 50 mg twice daily (the higher dose being used in subjects over 85 kg) |
What Should Be Done In Case Of Increase In Lung Function And Symptoms?
- Mild deterioration in lung function and symptoms should not lead to prompt discontinuation. However, if there is worsening of symptoms, a dose reduction or withdrawal may be deemed necessary.3
- If in doubt, lung functions with and without beta-blockers can be measured repeatedly before chronic use.3 The majority of patients with heart failure and COPD can safely tolerate beta-blocker therapy.3
What Are The General Risks With The Initiation Of Beta-Blocker Therapy?
The initiation of beta-blocker therapy can produce four types of adverse reactions that may warrant attention: fluid retention and worsening heart failure, fatigue, bradycardia or heart block, and hypotension.40
The incidence of fluid retention or worsening HF is not generally a reason for the permanent withdrawal of treatment. Such patients usually respond positively to intensification of usual therapy, and once treated, they remain excellent candidates for long-term beta-blocker treatment.40
Fatigue is multifactorial and probably the hardest symptom to confidently address. Even though it may be linked to beta-blockers, clinicians can reflect on other causes of fatigue such as sleep apnea, over-diuresis, or depression.40
If there is dizziness or lightheadedness along with bradycardia or if there is a second- or third-degree heart block, clinicians should reduce the dose of the beta-blocker.40
The risk of hypotension may be minimized by administering the beta-blocker and ACE inhibitor at different times during the day. Hypotensive symptoms may also resolve after a decrease in the dose of diuretics in patients who are volume-depleted. If it is accompanied by other clinical evidence of hypoperfusion, clinicians should reduce or discontinue beta-blocker therapy pending furtherevaluation.40
Should Beta-Blockers Be Given In COPD Patients With A History Of Asthma?
- A history of asthma should be considered as a relative contraindication to the use of any beta-blocker.20
What Do The Guidelines Recommend On The Use Of Beta-Blockers In Heart Failure Patients With COPD?
- NICE guidelines, 2010: Offer beta-blockers licensed for heart failure to all patients with heart failure due to left ventricular systolic dysfunction, including patients with COPD without reversibility.39
- ESC guidelines, 2012: COPD is not a contraindication for the use of a betablocker. A selective beta1 adrenoceptor antagonist (i.e. bisoprolol, metoprolol succinate or nebivolol) is preferred.20
- ACCF and AHA guidelines, 2013: Beta-blockers may be considered in patients who have reactive airway disease or asymptomatic bradycardia, but should be used cautiously in patients with persistent symptoms of either condition.40
- GOLD guidelines, 2015: Treatment with selective beta1-blockers is considered safe for heart failure patients who also have COPD. The benefits of selective beta1-blocker treatment in HF clearly outweigh any potential risk associated with treatment even in patients with severe COPD.41
What Precautions Should Be Taken When Prescribing Beta2-Agonists In COPD Patients With Heart Failure?
- Beta2-agonists should be preferably prescribed for clear relief of symptoms, after cautious consideration of the aetiology of breathlessness and objective documentation of the airflow limitation.38
- Avoid the use of oral beta-agonists, and minimize the dose and frequency of nebulized drugs.38
- In patients with heart failure and concurrent COPD requiring regular long-acting inhaled bronchodilators, treatment should preferably be initiated with long-acting anticholinergics rather than long-acting beta-agonists.38
What Precautions Should Be Taken When Prescribing Steroids In COPD Patients With Heart Failure?
- Oral steroids cause retention of sodium and water, which can potentially worsen the heart failure. However, this is likely with long-term use and, generally, does not occur with pulsed dosing for 7 to 10 days. Hence, it is preferable to avoid long-term use of oral steroids.6
- There are, as yet, no reports on inhaled corticosteroids being associated with cardiovascular problems.6
What Should Be Done In Patients Truly Intolerant To Beta-Blocker Therapy?
- Ivabradine (when the heart rate is ≥70 beats per minute) or digoxin may be considered in patients unable to tolerate beta-blocker therapy for reducing the risk of heart failure hospitalization.20
Conclusion
Though commonly seen, CHF is frequently unrecognized in COPD. The combination presents many diagnostic challenges with similar signs and symptoms, and overlapping risk factors such as smoking and common pathophysiological pathways.6, 14 Evaluation of natriuretic peptides is beneficial in selecting patients for echocardiography - the confirmatory test for heart failure. In patients with COPD, the presence of heart failure is linked with poor prognosis.
Treatment of heart failure in COPD essentially remains the same as that for those patients without COPD, including cardiovascular beta-blockers.6 There is evidence that these agents reduce exacerbations, improve survival, and do not adversely impact the lung function and symptoms in COPD.29, 30 Hence, the presence of COPD (even moderate or severe) should not serve as a contraindication for the use of cardio-selective beta-blockers in treating heart failure.13
Abbreviations and Acronyms
COPD: Chronic Obstructive Pulmonary Disease
CHF: Chronic Heart Failure
FEV1: Forced Expiratory Volume in first second
BNP: type B Brain Natriuretic Peptide
NT-proBNP: N-terminal pro type B Brain Natriuretic Peptide
ECG: Electrocardiography
RAS: Renin–Angiotensin System
ARIC: Atherosclerosis Risk in Communities study
ESC: European Society of Cardiology
ACCF: American College of Cardiology Foundation
AHA: American Heart Association
NICE: National Institute for Health and Care Excellence
SD: Standard Deviation
GOLD: Global Initiative for Chronic Obstructive Lung Disease
References
- J Assoc Physicians India. 2012; 60 Suppl:31-37
- A3674, Presented at the American Thoracic Society (ATS) Conference 2012
- Eur Heart J 2009; Suppl 1:A21–A25
- Pulm Pharmacol Ther 2010; 23:1–8
- Ann Epidemiol 2006; 16:63–70
- Eur Respir Monogr 2013; 59:50–63
- Chest 2005; 128:2005–2011
- World J Cardiol 2010; 2:305–307
- Arch Intern Med 2001; 161:996–1002
- Int J Chron Obstruct Pulmon Dis. 2013; 8:305–312
- Eur J Heart Fail 2012; 14:414–422
- Eur J Heart Fail 2006; 8:706–711
- J Thorac Dis 2012; 4:310–315
- Eur J Heart Fail 2009; 11:130–139
- Eur Heart J 2005; 26:1887–1894
- Curr Med Chem. 2007; 14:1121–1128
- Eur J Heart Fail 2009; 11:1182–1188
- Am J Cardiol 2008; 101:353–358
- Chest 2006; 130:1220–1230
- ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur Heart J. 2012; 33:1787–1847
- BMC Pulmonary Medicine 2012; 12:48
- Prim Care Respir J 2005; 14:236–241
- Trends Pharmacol Sci. 2011; 32:206–212
- Int J Chron Obstruct Pulmon Dis. 2007; 2:535–540
- J Clin Hypertens. 2009; 11:369–375
- J Am Coll Cardiol 2004; 44:497–502
- Eur Heart J 2003; 24:464–474
- Am J Respir Crit Care Med 2008; 178:661–666
- Arch Intern Med. 2010;170:880–887
- Cochrane Database of Systematic Reviews 2005; 4:CD003566
- BMJ 2011; 342:d2549
- Am J Respir Crit Care Med 2008; 178:695–700
- Int J Chron Obstruct Pulmon Dis. 2009; 4:177–183
- Clin Drug Invest 2002; 22:361–367
- Am Heart J 2007; 153:82.e1e82.e11
- Respir Med 2011; 105 S1:S44–S49
- Thorax 2012; 67:977–984
- J Am Coll Cardiol. 2011; 57:2127–2138
- Chronic Heart Failure. National Clinical Guideline For Diagnosis And Management In Primary And Secondary Care, updated 2010
- 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, Circulation 2013; 128:e240–e32
- Global strategy for the diagnosis, management and prevention of COPD by the Global initiative for Chronic Obstructive Lung Disease (GOLD), updated 2015