The patient showed remarkable improvement at follow-up at 8 months and now works fulltime.
The patient stopped pirfenidone in August 2011.
Pirfenidone is a novel anti-fibrotic drug used in the treatment of IPF and reduces the production of
other mediators of fibrogenesis, such as fibronectin and connective tissue growth factor. This case
report shows that the drug might have a beneficial role in bleomycin- induced lung disease and
warrants further clinical trials in this aspect.
P.N. Chhajed, A. Kate, H.S. Sandeepa et al. (Mumbai) Am J Respir Crit Care Med 185; 2012:A5907
IPF is a progressive fibrotic lung disease with an overall poor prognosis. One of the most prominent
symptoms of IPF is a persistent, non- productive, disabling cough for which there is also no
effective treatment.
M.R. Horton et al. in Baltimore, USA, investigated the role of thalidomide in suppressing cough in
IPF.
Thalidomide has potent immunomodulatory properties that make it an attractive candidate as a
therapeutic agent for IPF, based on the hypothesis that thalidomide would abrogate the cough by
suppressing inflammatory-induced sensory fiber activation within the respiratory tract.
The primary objective of this Phase III, double-blinded, randomized, placebo-controlled, crossover
trial was to determine the efficacy of 50?100 mg of thalidomide, administered daily for 12 weeks, to
suppress the chronic cough in patients with IPF. The primary outcome was the Cough Specific Quality
of Life Questionnaire (CQLQ). Secondary outcomes included the St. George's Respiratory Questionnaire
(SGRQ) and a visual analog scale (VAS) of cough severity. Normally distributed variables were
compared using paired t-tests, while other comparisons were performed with the Wilcoxon signed-rank
test. A p-value <0.05 was considered statistically significant.
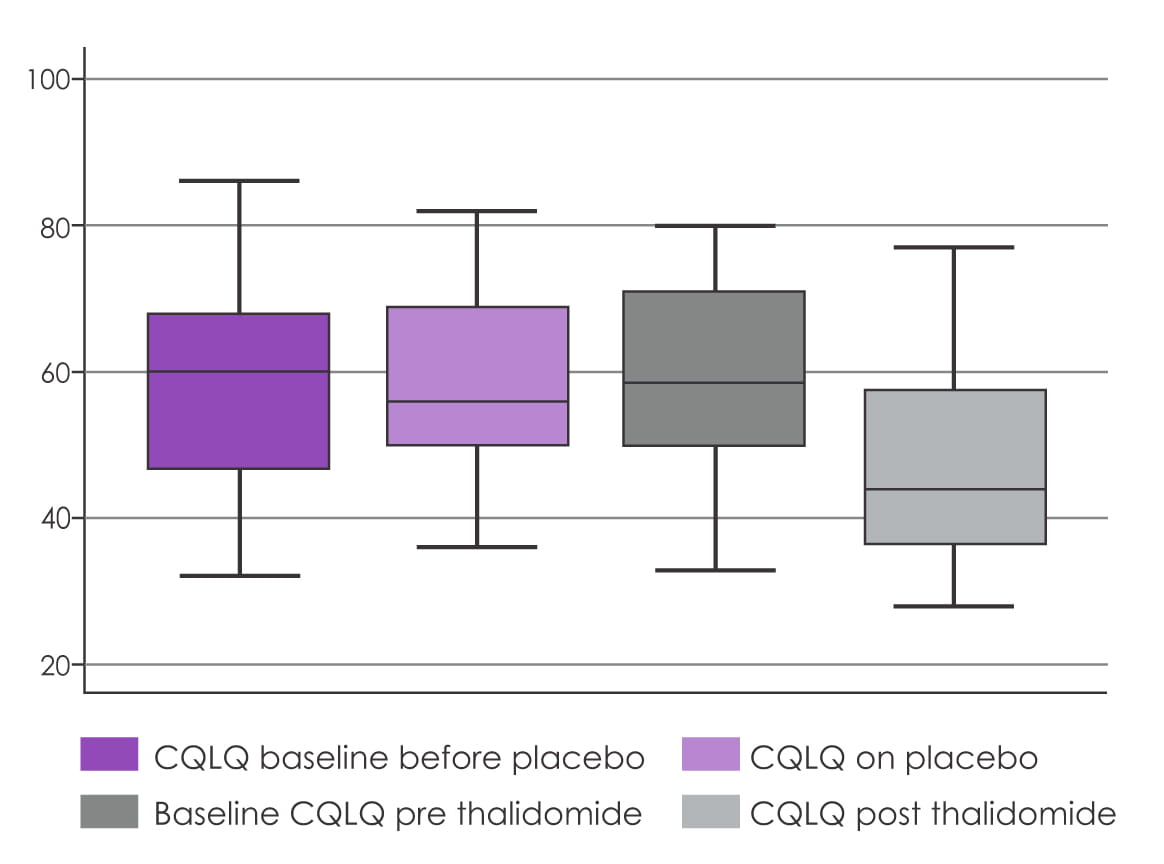
Of the 23 subjects enrolled and randomized, 20 finished the trial. The mean CQLQ worsened by
1.13±1.65 (p=0.5) with placebo and improved by 10.0±2.3 (p=0.0004) with thalidomide.
Results showed that thalidomide significantly improved cough as measured by the CQLQ. Thalidomide
significantly improved the cough VAS by 42.3±4.45 points (p<0.0001) and well as
significantly improving the respiratory quality of life as measured with the SGRQ (total SGRQ
decreased by 10.8±3.7, p=0.009).
Hence, thalidomide inhibits cough and improves the quality of life in patients with IPF, and this
study identifies, for the first time, an effective pharmacologic treatment for cough in IPF. Future
studies need to be carried to assess if the anti-inflammatory effects of thalidomide may also
positively impact disease course and survival.
M.R. Horton, V. Santopeitro, L. Matthew et al. (Baltimore, USA) Am J Respir Crit Care Med 185;
2012:A363
Hypersensitivity pneumonitis (HP) is a frequent interstitial lung disease, with diagnosis being
difficult and often relying on histopathology. Persistent exposure to the allergen may lead to
chronic illness, resulting in end-stage lung fibrosis with a high mortality rate.
Earlier data has shown that morphological features of UIP-like and nonspecific interstitial pneumonia
(NSIP)-like patterns are associated with poor outcomes. Recent reports indicate that HRCT may be
able to identify similar patterns, but the prognostic implications in HP have not been elucidated.
H. Mateos et al. from Mexico evaluated mortality between the different HRCT patterns of sub-acute and
chronic HP in 56 consecutive HP patients over a period of 5 years.
The time "0" was the date of diagnosis, and the outcome was the date of the last appointment, or
death. The HRCT studies were evaluated by two blinded, experienced observers. The cases were divided
in two groups: 1) typical CT pattern of sub-acute or chronic HP (45 patients); and, 2) non-typical
CT pattern of HP (UIP-like, NSIP- like: 11 patients).
Results showed that the mean age was 45±10 years, and the majority of patients (91%) were
women. In the whole group, 5-year mortality was 32%, with a median survival of 8 years. The higher
the fibrosis score on HRCT, the greater the tendency to be associated with increased mortality
(p=0.09). Also, patients with a non-typical HRCT pattern showed significantly higher 5-year
mortality compared to patients with a typical HRCT pattern (77% versus 27%).
H. Mateos, M. Mejia, I. Buendia et al. (Mexico) Am J Respir Crit Care Med 185; 2012:A1583
IPF, the most common interstitial lung disease of unknown etiology, has a poor prognosis. Earlier
evidence suggests that female gender is associated with improved survival and less rapid progression
of disease in IPF. Pulmonary hypertension is one of the co-morbidities known to influence mortality
in IPF.
Data from earlier studies show that the right ventricular systolic pressure via 2D ECHO is greater in
men than women with IPF. Accordingly, in the COMET trial, M.K. Han et al. assessed if the pulmonary
artery (PA) diameter, which is known to correlate with pulmonary hypertension, would be greater in
men than women.
The HRCT scans from the COMET study were analyzed in all newly diagnosed IPF patients as outlined by
the ATS/ERS guidelines involving analysis of HRCT and open lung biopsy, where appropriate. A single
HRCT interpreter was blinded to the patients' demographics and clinical characteristics, and
measured the diameter of the main PA at the level of bifurcation. Data on 18 women and 40 men were
available for comparison. Mean FVC%- and DLCO%-predicted was comparable among both sexes.
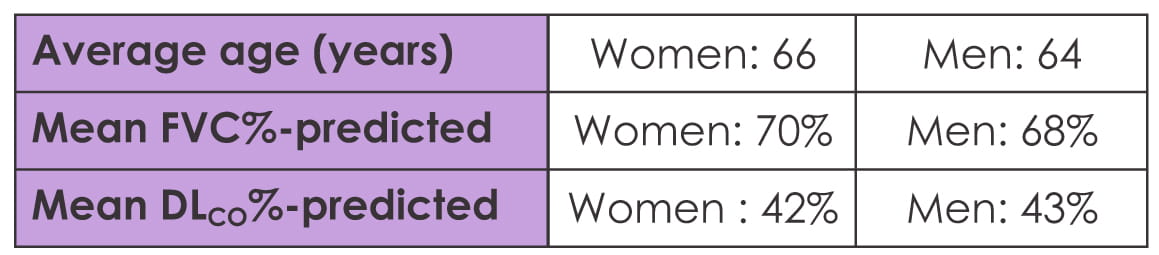
However, mean PA measurement was significantly more in men compared to women; it was 3.12 cm and 2.91
cm for women (p=0.045) even after adjusting for confounders like body surface area (p<0.001).
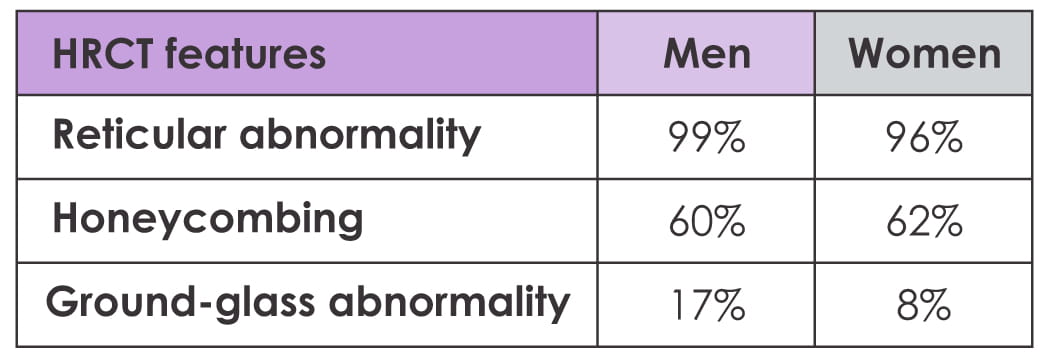
A cutoff of 3.32 cm has been suggested as a criterion for pulmonary hypertension, and 50% of men
versus 2% of women met this criterion (p=0.048). No significant difference was seen in other HRCT
features.
Hence, in line with prior findings, CT evidence of pulmonary hypertension suggests that its
prevalence is greater in men than women, suggesting a possible mechanism for worse prognosis in
males with IPF.
M.K. Han, N. Tayob, S. Murray et al. (Michigan, USA) Am J Respir Crit Care Med 185; 2012:A4466
There is a lack of reliable data on the role of screening auto- antibodies in patients with
idiopathic interstitial pneumonia. In this study, B.H. Kang et al. investigated the incidence of new
connective tissue disease in patients with idiopathic interstitial pneumonia and the predictive role
of auto-antibodies in its development.
Retrospective baseline and follow-up data of 696 patients with idiopathic interstitial pneumonia
(IPF: 534; NSIP: 85; cryptogenic organizing pneumonia: 77) at one single tertiary referral center
were reviewed.
Median follow-up period was 35.3 months.
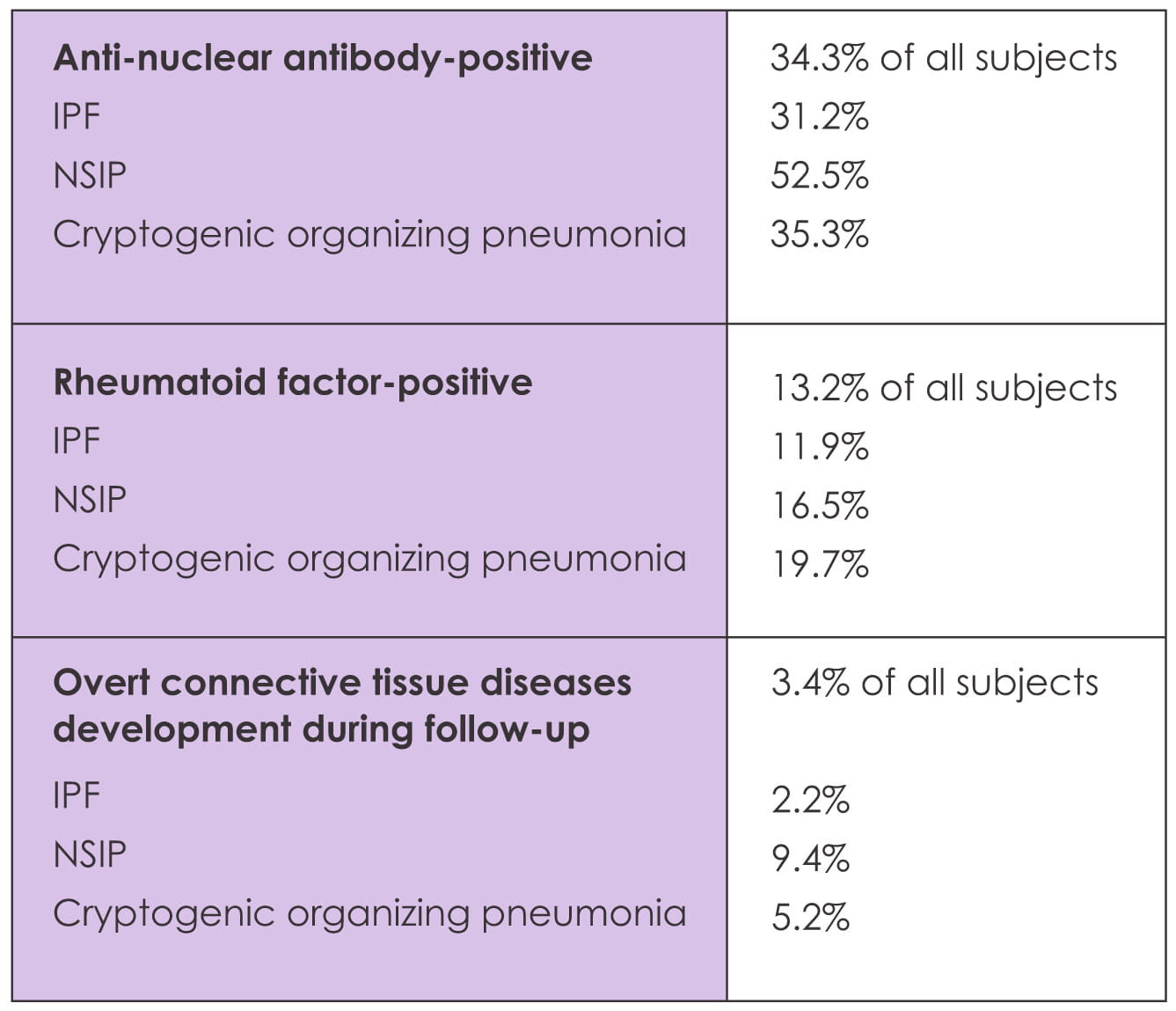
The majority (75%) of connective tissue disease patients had a positive anti-nuclear antibody at
initial diagnosis.
The frequency of new connective tissue disease was significantly higher among the patients with
positive anti-nuclear antibody (all: 8.0% versus 1.4%, p<0.001; IPF: 5.7% versus 0.9%, p=0.002)
and positive rheumatoid factor (10.6% versus 2.7%; p=0.002) compared to negative auto-antibody. Also
the incidence of connective tissue disease was significantly higher with anti-nuclear antibody titer
higher than 1:320.
Hence, in this study, anti-nuclear antibody was a significant predictor for new connective tissue
disease development although the incidence was low, especially in IPF.
B. H. Kang, J. Park, J. Roh et al. (Korea) Am J Respir Crit Care Med 185; 2012:A6613
Pirfenidone is a recent anti-fibrotic drug, approved in India, Japan and Europe for IPF-UIP. However
there is insufficient Indian data on the tolerability of pirfenidone.
P. N. Chhajed et al. from Mumbai undertook the current observational study to assess the tolerability
of pirfenidone in IPF-UIP in 30 consecutive patients with a clinical-radiological diagnosis of
IPF-UIP initiated on pirfenidone.
Pirfenidone was initiated at a dose of 200 mg thrice a day and titrated to a maximum dose of 400 mg
thrice a day over 2 to 4 weeks. In the case of 2 patients, they were started on 400 mg three times
daily.
Baseline liver function tests were performed, and lung function and 6-minute walk tests were possible
in 22 patients. Patients were followed up initially at 2 to 4 weeks, then monthly/quarterly or as
required clinically. In addition, all patients were initiated on prednisolone 10 mg/day,
N-acetylcysteine 1,800 mg/day and a proton-pump inhibitor. Baseline liver function was normal in all
patients.
Baseline characteristics
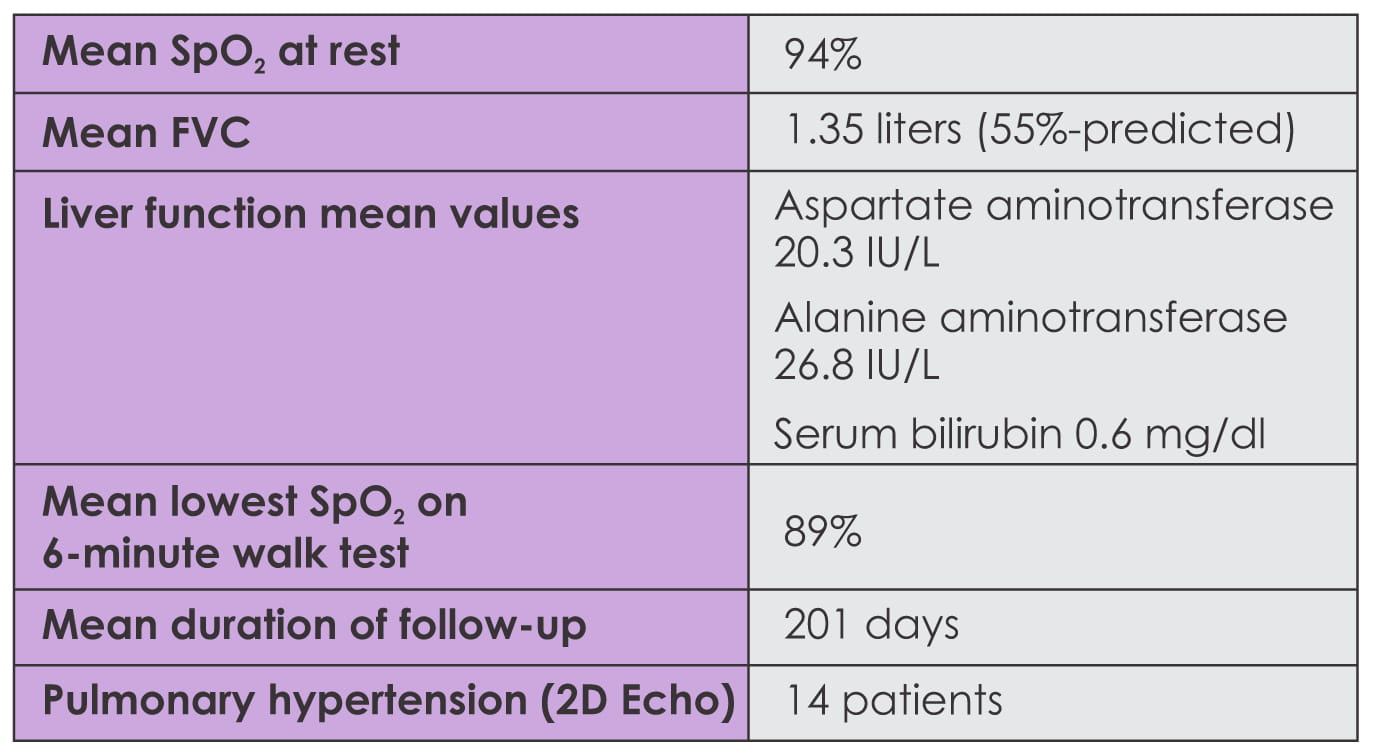
- There was no significant increase in liver enzymes at follow-up.
- The dose of pirfenidone was increased to 1,200 mg/day in 17 patients; however, it was not possible
in 11 patients due to gastrointestinal side effects (nausea/vomiting, 10 patients, loose motions, 1
patient).
- Discontinuation: Pirfenidone was stopped in 4 patients because of
skin itching and rash (3 patients 600 mg/day; 1 patient 1,200 mg/day)
- There were 3 patients who continued to take pirfenidone, despite skin itching (no skin
discoloration) and counseling about side effects, and took symptomatic treatment
- During the observational period, 3 patients expired.
Hence, it was observed that skin itching and rashes do not seem to be dose-related.
Gastrointestinal side effects may be dose-related and necessitate administration of lower doses
in patients with IPF-UIP. Presently, the benefit?risk ratio on whether administration of a lower
dose of pirfenidone is better than not utilizing it as an effective treatment for IPF patients
is unclear.
P.N. Chhajed, H.S. Sandeepa, P. Chaudhari et al. (Mumbai). Am J Respir Crit Care Med 185;
2012:A4502.
BIBF 1120 is a novel triple angiokinase inhibitor that blocks the intracellular signaling
pathways of PDGF/R, FGF/R, and VEGF/R. In the earlier results of the TOMORROW trial, treatment
with BIBF 1120 300 mg/day for 1 year reduced a decline in lung function in IPF patients. Recent
data suggests that the quality of life reflected by patient-reported outcomes may improve with
BIBF 1120 as reported by K.K. Brown from Denver, USA, and other TOMORROW trial investigators.
The TOMORROW trial was a 12-month, Phase II, double-blind trial that investigated the safety and
efficacy of BIBF 1120 in 432 patients with IPF who were randomized to receive one of four doses
of BIBF 1120 (50 mg, 100 mg, 200 mg or 300 mg/day) or placebo. Patient-reported outcomes were
assessed using the SGRQ (St. George's Respiratory Questionnaire) and the Medical Research
Council (MRC) dyspnea scale, and through collection of data on spontaneously reported cough and
dyspnea.
The SGRQ and MRC were assessed at baseline and 6, 12, 24 and 52 weeks after the start of
treatment. Spontaneously reported adverse events were documented throughout treatment.
At 12 months, the SGRQ total score improved by ? 0.79, ? 3.28, ? 3.98 and ? 6.12 points relative
to placebo for rising BIBF 1120 dose groups and was significant for 300 mg/day dose (p=0.0071
for 300 mg/day versus placebo). Compared with placebo, 300 mg/day of BIBF 1120 achieved
improvements of ? 9.6 points for the SGRQ symptoms domain (p=0.0028), ? 7.16 points for the
activity domain (p=0.0043), and ? 4.35 points for the impacts domain (p=0.0948).
The MRC dyspnea score in decreased in 9 patients taking 300 mg/day versus 6 on placebo, and
increased in 26 patients taking 300 mg/day versus 31 on placebo (odds ratio 1.551; p=0.17). Mean
change in the SGRQ total score correlated with a mean change in FVC from baseline (r = ? 0.304;
p=0.0081). Change from baseline in the SGRQ activity domain was unrelated to change in body
weight (r = ? 0.05; p=0.6460).
A significant difference was seen between the 300 mg/day dose and placebo when the worst value
for the SGRQ total score was used for patients who discontinued treatment prematurely
(p=0.0131). Also, fewer spontaneous reports of dyspnea (7.1% versus 12.9%) and cough (9.4%
versus 20.0%) were noticed in the 300 mg/day group versus placebo.
Table 1: Change in patient-reported outcomes from baseline to 1 year
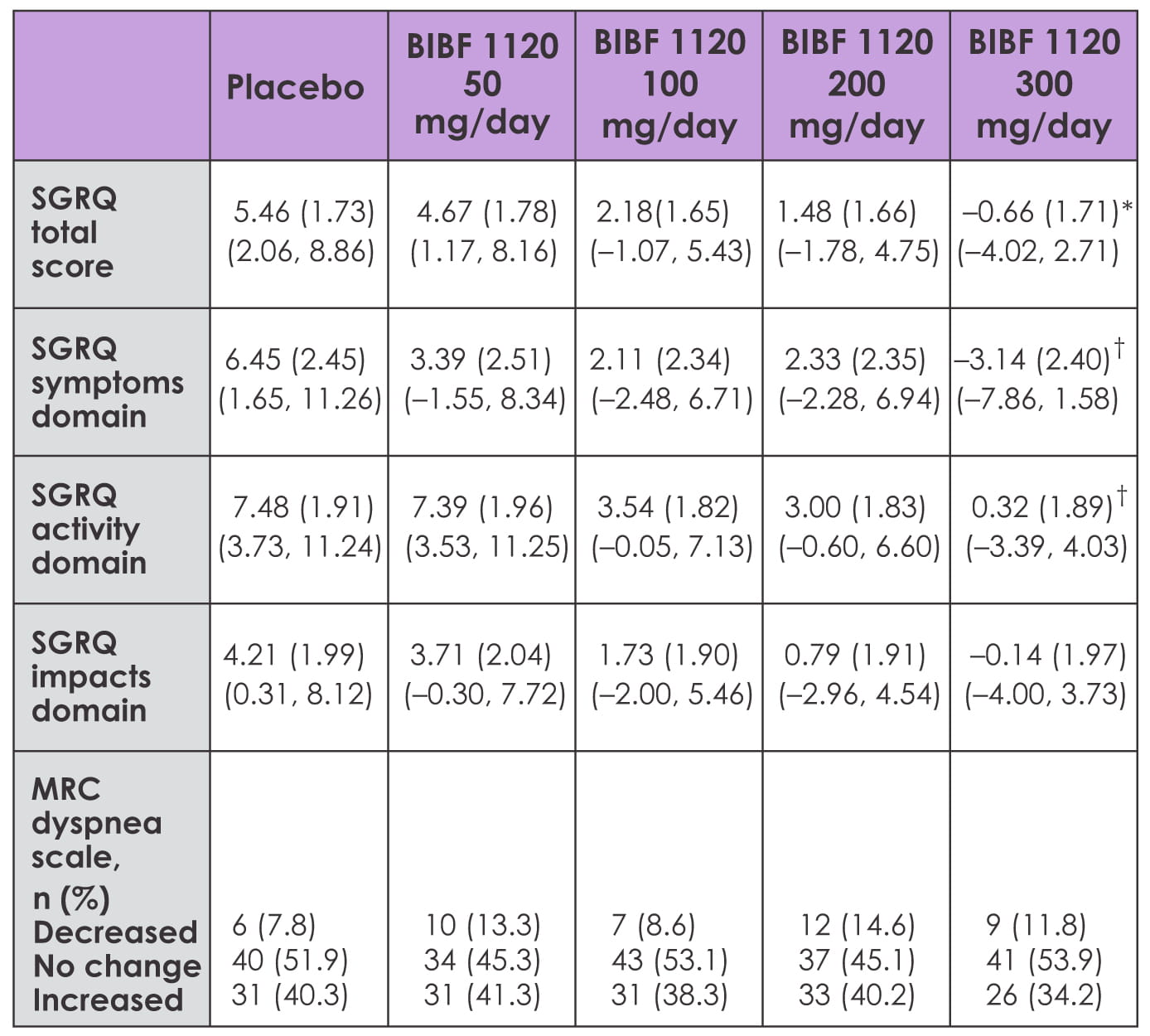
Hence, in IPF patients, treatment with BIBF 1120, 300 mg/day over 1 year, preserves the quality
of life reflected by patient-reported outcomes, and may decrease the symptoms of dyspnea and
cough, compared with placebo. This may be correlated with a reduced decline in lung function.
K.K. Brown, L. Richeldi, U. Costabel et al. (Denver, USA) Am J Respir Crit Care Med 185;
2012:A3634.