SMART is a type of treatment regimen in asthma in which the inhaled corticosteroid (ICS)/long-acting beta2 -agonist (LABA) combination of budesonide/formoterol can be used as not just daily maintenance treatment but also as rescue medication during increased symptoms.
SMART Therapy...
Your Questions Answered
What is SMART Therapy?
How Does an Exacerbation Evolve?
Exacerbations give us a fairly good idea of the level of asthma control and, hence, to understand the concept of SMART better, we will first have to understand exactly how an exacerbation evolves.
Clinical Evolution of an Exacerbation
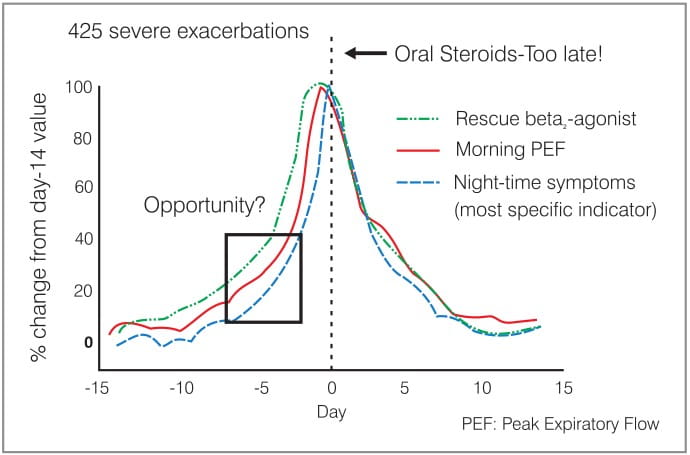
The analysis of the 425 exacerbations in the FACET* trial revealed that the exacerbations were characterized by a gradual decline in the peak expiratory flow (PEF) over 5 to 7 days, followed by a more rapid fall over 2 to 3 days. The increase in symptoms and the use of a rescue beta2-agonist were similar in pattern to the fall in the PEF. One of the goals in asthma therapy is to prevent exacerbations. Knowing the rate of change in the PEF and the symptoms as an exacerbation develops might help to determine whether the exacerbations can be identified at an early stage. This would enable treatment to be started earlier - thereby reducing the severity of the exacerbation, and this is the idea behind the SMART regimen (Figure 1).
{ * FACET: The Formoterol and Corticosteroids Establishing Therapy trial, was a double-blind, randomized, parallel group trial with 852 patients, who had been on ICS before the start of the study, receiving either low-dose budesonide (100 mcg), low-dose budesonide plus formoterol (12 mcg), high-dose budesonide (400 mcg) or high-dose budesonide plus formoterol (12 mcg) twice daily along with a short-acting beta2-agonist (SABA) as rescue medication. The primary endpoint was the incidence and the rate of severe and mild exacerbations. The study concluded that in patients who have persistent symptoms of asthma despite treatment with ICS, the addition of formoterol to budesonide may be beneficial in improving asthma control.}
Evolution of an Exacerbation in the Airways
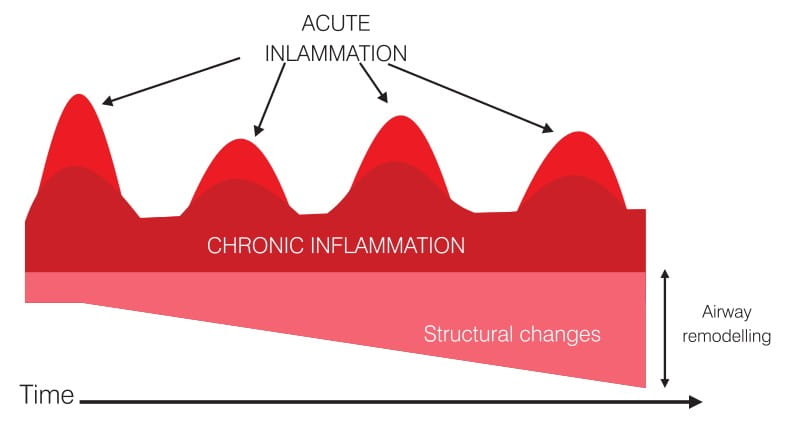
Asthma is a chronic inflammatory disease with variable episodic attacks. Chronic inflammation in asthma is associated with sub-epithelial fibrosis, smooth muscle hyperplasia/hypertrophy, mucous gland hyperplasia and neo-vascularization. On the entry of an allergen or some other trigger, there is a substantial increase in this chronic inflammation (Figure 2).
This acute inflammation in asthma is associated with bronchoconstriction, plasma exudation/oedema, vasodilatation and mucus hypersecretion, all of which are the pathophysiological features of an asthma exacerbation. The clinical result of these events is that the patient experiences increased breathlessness, coughing, wheezing and chest tightness. The severity of the exacerbation is dependent on the extent of this acute inflammation and, hence, it is essential to treat this acute inflammation.
If asthma remains uncontrolled or poorly controlled, the underlying persistent inflammation in the airways leads to structural changes (remodelling) that reduce the extent of airway response to the therapy.
What is the SMART Approach?
The traditional asthma treatment model involves the use of ICS or an ICS/LABA combination used with a SABA for relief.
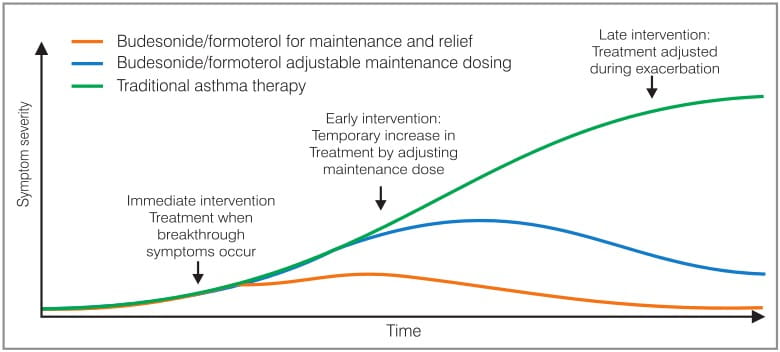
Figure 3 shows the difference between the traditional fixed dosing, adjustable maintenance dosing (AMD) and SMART.
Why is it Possible to Use SMART as Maintenance and Reliever Therapy?
The combination of budesonide/formoterol contains the LABA, formoterol, and the ICS, budesonide. These have different modes of action and show additive effects in terms of reduction of asthma exacerbations. The specific properties of these two components allow the budesonide/formoterol combination to be used as a maintenance treatment for asthma, or as both maintenance and reliever therapy.
The difference between the single combination inhaler and a SABA as relievers is the simultaneous administration of an ICS, which may play a key role in reducing exacerbations. There have been studies that have shown that single reliever therapy along with the combination inhaler as rescue therapy was similar to using the same fixed-dose combination therapy with formoterol alone as rescue therapy in terms of the number of additional puffs needed to control symptoms; also, the combination inhaler was significantly better than using formoterol alone as reliever in reducing exacerbations, thereby providing strong support for the idea that the "as required" additional use of an ICS plays a critical role in reducing asthma exacerbations and improving asthma control.
Why is SMART Possible with Budesonide/Formoterol?
When inhaled, budesonide exerts a dose-dependent anti-inflammatory action in the airways, resulting in reduced symptoms and fewer asthma exacerbations. Budesonide is retained in the airway tissue to a greater extent than other ICS and also has the unique property of undergoing esterification, thus increasing its retention and thereby prolonging its duration of action. There is also increasing evidence that ICS have a relatively rapid suppressive effect on inflammation.
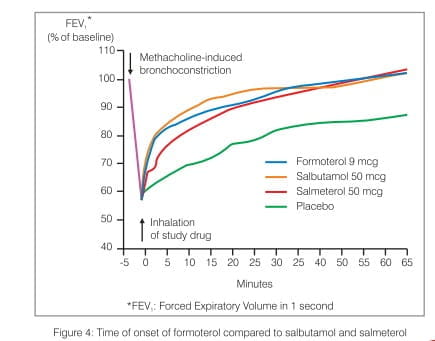
Inhaled formoterol, a selective LABA bronchodilator, results in rapid and long-acting relaxation of bronchial smooth muscle in patients with reversible airways obstruction. The bronchodilating effect is dose-dependent, with an onset of effect within 1-3 minutes. Studies performed in both stable and acute severe asthma cases confirm that formoterol works as fast as salbutamol or terbutaline and faster than salmeterol (Figure 4).
The duration of effect is at least 12 hours after a single dose. One limitation of using formoterol alone as a reliever is the concern that increasing doses of LABA would provide bronchoprotection against smooth muscle contraction but still allow increases in inflammation to go unchecked. To counter this problem, it was suggested that a combination of budesonide/formoterol, developed initially as a maintenance therapy, could also be used for relief of symptoms, i.e. SMART.
With SMART, patients take a regular maintenance dose of the budesonide/formoterol combination and additional inhalations of the same as needed. When additional inhalations are required, there is an increased dose of both components, thereby addressing the underlying inflammation as well as offering effective symptom relief with every inhalation.
SMART can replace the use of a SABA as it works as fast and effectively as salbutamol in relieving severe bronchoconstriction (Figure 5).
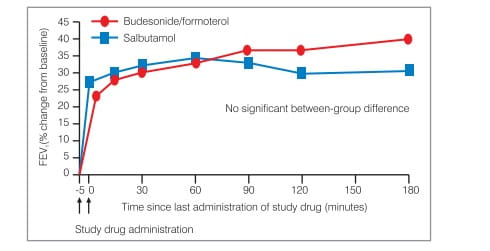
This rapid onset of effect means that patients feel the benefit of the budesonide/formoterol combination very quickly-patients have confirmed that they feel relief from breathlessness within the first minute of taking the puff (Figure 6).
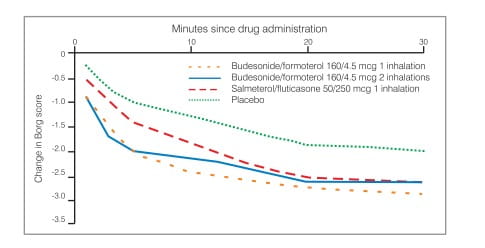
SMART aims to provide a dose of medication that is appropriate to the level of inflammation, treating the underlying inflammation with every inhalation. Furthermore, increasing the dose of budesonide/ formoterol at the first sign of asthma symptoms may prevent the development of exacerbations.
What Makes SMART Work?
The exact mechanism for the beneficial effects of budesonide/formoterol SMART has yet to be fully elucidated. Budesonide and formoterol are reported to have a synergistic relationship whereby their combined effects are complementary and additive, thereby resulting in the efficacy of this treatment approach.
Single doses of budesonide/formoterol protect against late-phase bronchial hyper-responsiveness provoked by allergens; this bronchoprotective/airway stabilizing effect was not seen with budesonide and formoterol monotherapies.
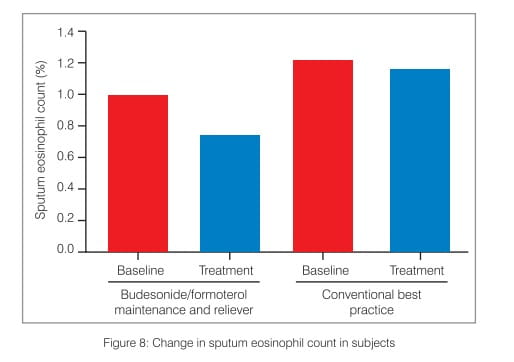
In a study conducted in a subgroup of patients to examine the changes in induced sputum eosinophil count in patients receiving SMART (160/4.5 mcg b.i.d.) compared with the conventional best practice, it was concluded that budesonide/formoterol maintenance and reliever therapy achieved similar control of eosinophilic inflammation compared with conventional best practice, despite a lower mean ICS dose (Figure 8).
In Which Type of Patients Should SMART be Used?
This treatment approach should be considered in any patient who requires combination treatment with an ICS/LABA, especially those patients who are prepared to use a reliever in response to relevant symptoms.
What is the Evidence Regarding the Efficacy of SMART in Literature?
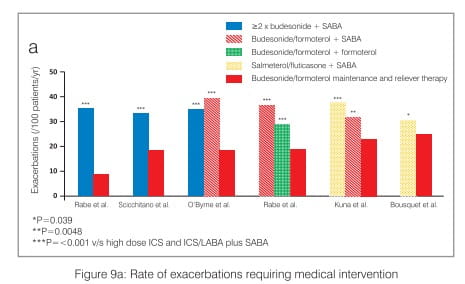
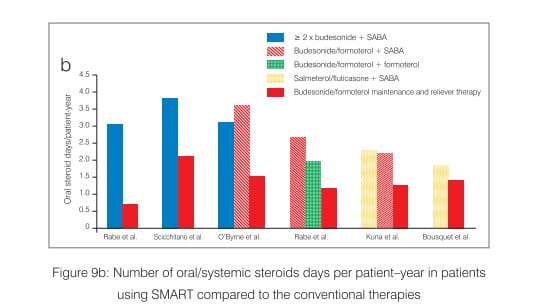
Large-scale, randomized, blind clinical studies in patients with asthma, aged 12 years or older, have highlighted the benefits of the budesonide/formoterol maintenance and reliever therapy. These studies have consistently shown significant improvements in the time to first exacerbation and reductions in the rate of severe exacerbations (defined as events requiring oral corticosteroids for 3 days or hospitalization/emergency room treatment) with budesonide/ formoterol maintenance and reliever therapy as well as reductions in the total number of oral steroid days relative to all comparator therapies (Figure 9 a and b).
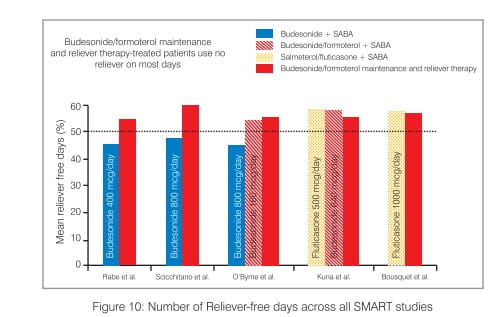
The mean number of days requiring high reliever medication has been shown to be lower with SMART compared to traditional therapies using SABAs (Figure 10).
Is SMART Safe?
Randomized clinical studies have demonstrated that, compared with conventional therapies, SMART has a favourable tolerability profile and similar side effects; most adverse events are mild to moderate and no new or unexpected adverse events have been reported.
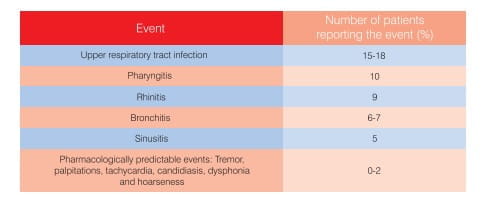
The incidence, frequency and profile of adverse effects were similar to comparator therapies, and discontinuations because of adverse effects were lower or equivalent. The most commonly reported adverse events were as follows:
A recent study analysed safety data from a trial database (N= 14,346) comprising six randomized clinical trials where budesonide/formoterol was used as maintenance and reliever therapy for at least 6 months and it concluded that budesonide/formoterol maintenance and reliever therapy was well-tolerated and not associated with increased risk of death or cardiac-related serious adverse events or discontinuation, compared with fixed-dose alternatives. Furthermore, asthma-related serious adverse events and discontinuations were significantly reduced.
A possible concern is that the use of SMART may deliver a higher overall ICS dose, resulting in ICS-related side effects. However, SMART has been shown to achieve similar efficacy to conventional therapies using a similar or even lower overall ICS load.
Do Any Guidelines Recommend SMART Therapy?
Budesonide/formoterol maintenance and reliever therapy is recommended by the Global Initiative for Asthma Management (GINA) guidelines for the treatment of patients with moderate-to-severe disease that is uncontrolled with ICS.
Additionally, the country-specific guidelines such as those of the Canadian Thoracic Society (CTS) also recommend that "formoterol should only be used as a reliever in individuals on regular ICS therapy in adults and children 12 years of age and over, preferably in the same inhaler device (i.e. combination budesonide/formoterol preparation), instead of two separate inhalers".
Also, the British Thoracic Society guidelines state that "in selected adult patients at step 2 and 3 who are poorly controlled, the use of SMART as rescue medication instead of a SABA, in addition to its regular use as controller therapy, has been shown to be an effective treatment regimen".
Accordingly, budesonide/formoterol maintenance and reliever therapy has been approved in more than 90 countries, including those of the European Union.
How to Use SMART Therapy?
Two strengths of budesonide/formoterol SMART are approved for use only in adults, aged 18 years and over - 100/6 mcg and 200/6 mcg per inhalation allowing for personalization, dependent on clinician advice and patient response.
Maintenance doses of 1 inhalation twice daily or 2 inhalations once daily have been studied in moderate-to-severe asthma and both regimens have been shown to be highly effective. A maintenance dose of 2 inhalations twice daily is an option in patients with more severe asthma.
Patients should additionally take 1 inhalation, as needed, in response to symptoms. If symptoms persist after a few minutes, an additional inhalation should be taken. Not more than 6 additional inhalations should be taken on any single occasion.
A total daily dose of more than 8 inhalations is not normally needed; however, a total daily dose of up of 12 inhalations could be used for a limited period. Patients using more than 8 inhalations daily should be strongly recommended to seek medical advice. They should be reassessed and their maintenance therapy should be reconsidered.
Is SMART Useful in Children?
A single inhaler as maintenance and reliever therapy is not recommended in children and adolescents since it has not yet been approved for use in this age group. However, in a large randomized, controlled trial with 341 children (aged 4 to 11 years) [budesonide/formoterol 80/4.5 mcg o.d. maintenance plus relief], it was concluded that the SMART regimen reduced exacerbation rates compared with both the fixed-dose combination and higher fixed dose alone in children with asthma.
Regarding safety, the same study observed that patients receiving the SMART regimen grew significantly more than patients in the fixed-dose budesonide group, thus making the SMART regimen an effective and well-tolerated treatment approach that may greatly simplify paediatric asthma management in the future.
Are There Any Patients in Whom SMART Therapy Should Not be Used?
- Patients less suitable for SMART may include those who are prone to habitual and unnecessary high reliever use, such as patients with a diagnosis of asthma who use a reliever for dyspnoea due to unfitness, being overweight or because of some other underlying disease such as vocal chord dysfunction or even due to anxiety.
- Furthermore, physicians need to be aware of a small group of patients who may not perceive the symptoms or use too little reliever therapy, such as patients who are concerned about issues such as higher reliever cost per day or who have a fear of the effects of incremental ICS doses. For these patients, the most important factor may be optimizing the dosage of maintenance medication.
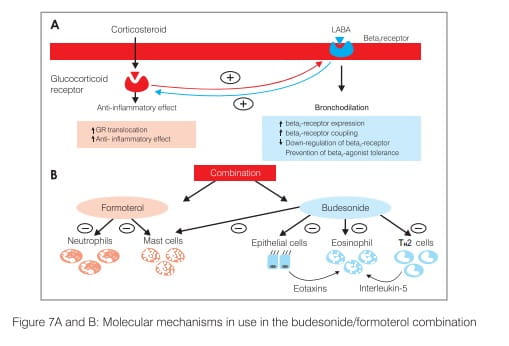
- Following binding to the glucocorticoid receptors (GR) in the cell cytoplasm, translocation to the cell nucleus leads to the activation of glucocorticoid response elements, recognition sites on the promoter regions of some genes that are activated by interaction with a GR (Figure 7A).
- In addition, formoterol has inhibitory effects on neutrophils and mast cells, whereas budesonide also inhibits mast cell function and also inhibits T-helper (TH2) cells and epithelial cells, resulting in reduced eosinophils (Figure 7B).
- In the traditional approach, the medical intervention comes into play much later when the exacerbation has developed and the condition of the patient is so severe that he has to be admitted to the emergency room/hospital.
- In the case of AMD, the intervention in the form of additional doses of maintenance doses is early, i.e. when a patient starts experiencing symptoms, he takes a reliever dose and increases the number of puffs of the maintenance dose.
- However, in SMART, the patient takes additional puffs of budesonide/formoterol as a reliever as soon as breakthrough symptoms occur (immediate intervention). This treatment strategy has been developed for moderate-to-severe asthma patients, which allows physicians to maximize the potential of the "window of opportunity" in preventing the progression and development of an exacerbation.
Can SMART be Used in Pregnant and Lactating Women?
During pregnancy and lactation, budesonide/formoterol should only be used after special consideration, particularly during the first 3 months and shortly before delivery. One study reported that in asthmatic women, maintenance treatment with budesonide (200 or 400 mcg b.i.d.) results in negligible systemic exposure of breastfed infants.
Can SMART be Used as Prophylaxis?
Reliever inhalations of budesonide/formoterol should be taken in response to asthma symptoms but are not intended for regular prophylactic use, e.g. before exercise.
How Long Should SMART be Continued?
Dosage is individual and should be adjusted according to disease severity. When control has been achieved, the dose should be titrated to the lowest effective dose, which could include once-daily use.
Is SMART Possible with any Other ICS/LABA Combination?
Due to its unique properties, the budesonide/formoterol combination is currently the only ICS/LABA combination that is approved to be used as maintenance and relief in one inhaler. This is because both budesonide and formoterol show a clear dose response (i.e. higher doses have a greater effect).
This approach is not possible with a combination product containing the LABA, salmeterol (such as salmeterol/fluticasone), due to the lack of dose response and slower onset of action for salmeterol.
Theoretically, all formoterol-containing ICS/LABA combinations can be considered for a SMART regimen and a number of other combinations using formoterol and other ICS are in development. However, there have been no trials conducted to demonstrate their effectiveness and safety if used as SMART.
A Re-look at the Dosing Guide in SMART
Two strengths of budesonide/formoterol SMART are approved for use only in adults, aged 18 years and over-100/6 mcg and 200/6 mcg per inhalation allowing for personalization, dependent on clinician advice and patient response.

References
- Eur Respir J 2002; 19:182-191.
- Eur Respir J 2007; 29:587-595.
- J Allergy Clin Immunol 1998; 101:457-463.
- Cochrane Database Syst Rev 2001; CD003271.
- Eur Respir J 1997; 10:2484-2489.
- Respir Med 1998; 92:1017-1021.
- Respir Med 2000; 94:607-611.
- Respir Med 2003; 97:1067-1074
- Drugs 2009; 69 (2): 137-150
- Allergy 2008; 63:1567-1580
- Global Initiative for Asthma Management (GINA) guidelines, updated 2010.
- British Thoracic Society (BTS) Guidelines, updated 2009.
- Canadian Thoracic Society Asthma Management Continuum - 2010 Consensus Summary for children, 6 years of age and over, and adults. Can Respir J 2010; 17(1):15-24.
- Pulm Pharmacol Therap 2010; 23:88-96.
- Chest 2006; 130:1733-1743.
- Eur Respir J 1999; 13:988-92.
- Pulm Pharmacol Therap 2006; 19:139-147.
- Pulm Pharmacol Therap 2004; 17:89-95.
- Eur Respir J 2008; 31:982-89.
- Am J Respir Crit Care Med 1999; 160:594-599.