Inhalation Devices: Current Status
Gaps in Current Inhalation Therapy
Inhalers have been available for over 60 years for the management of asthma and COPD; however, achieving optimal outcomes from the therapy are still far from desired. One of the key reasons has been attributed to improper inhaler technique.
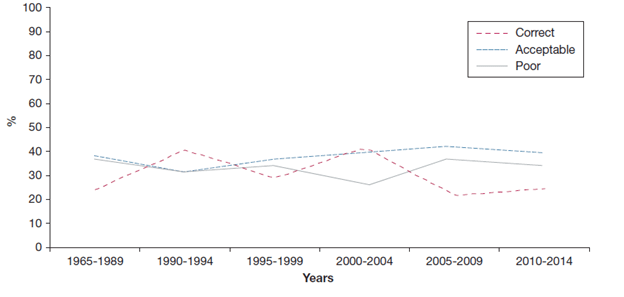
A recent review, aimed at assessing the most common errors in inhaler use over the past 40 years, analysed the data of around 54,354 subjects from 144 articles1
Based on the inhaler technique reported in the shortlisted articles, patients were categorized into three major categories: 1. Correct: when all the steps were performed in agreement; 2. Acceptable: when approximately 80% of those steps were correct and no critical error was observed; and, 3. Poor: when the researchers observed one or more critical errors and/or there were errors in more than 50% of the inhalation procedure steps (Figure 1).
The main finding was a high frequency of poor and/or suboptimal inhaler use for all types of devices. The pressurised Metered Dose Inhaler (pMDI) had the highest average frequency of errors (>40%). The researchers observed that the problem of incorrect or suboptimal use had not diminished over the past 40 years, even though considerable effort has been invested in education, training and device development.
The pMDIs are most popular due to their small size and unobtrusive nature; however, drug delivery is highly dependent on good inhaler technique and many patients find pMDIs difficult to use correctly. Over the last 60 years, numerous errors with pMDIs have been described, with poor hand-breath co-ordination being one of the most commonly reported errors.2 In a large (n=4,078) study, 71% of patients were found to have difficulty using pMDIs, and almost half of them had poor coordination.3In another study in patients with asthma or COPD (n=246), 81.4% of the pMDI users found difficulty in coordinating between actuation and inhalation.4 Some of these issues of pMDIs can be overcome by attaching a valve-holding chamber or a spacer or by using a Dry Powder Inhaler (DPI).
Various types of DPIs are available – single-unit dose, discreet multi-dose, and reservoir multi-dose. DPIs are breath-actuated and, hence, overcome the problem of actuation with inhalation. Despite this benefit, DPIs are also associated with a few difficulties. A systematic literature review5 revealed that up to 90% of patients failed to use their DPI correctly. One of the common errors made by patients with DPI, leading to insufficient drug delivery, was failure to inhale forcefully and deeply through the device. The need to inhale forcefully and, consequently, generate a sufficient inspiratory flow in DPIs suggests that using DPIs correctly could be a problem for children (below 5 years of age) or patients with severe airflow limitation.6 The shortcomings with current devices such as the pMDI, pMDI/spacer and DPI are listed in Table 1.
|
pMDI |
pMDI/Spacer |
DPI |
|
Many patients find pMDIs difficult to use correctly. Patients are often not able to coordinate actuation of the device with inspiration. Drug delivery is highly dependent on good inhaler technique. Some pMDIs do not have a dose counter, which makes it difficult to track remaining doses in the device.
|
Bulkier and less convenient than a pMDI alone. Most spacers are made from plastic material, which can build up electrostatic charge on their surface. These electrostatic charges can significantly reduce the amount of drug available for inhalation.
|
Most DPIs are inspiratory-effort-dependent, which can make using the device difficult for young and elderly patients, patients with severe lung impairment, and those with reduced inspiratory flow. Instructions for use vary among devices. Powders may be moisture-sensitive. Larger particle sizes may reduce drug delivery to areas of inflammation in the small airways. |
Understanding Inhalation Devices: The Patient and Physician Perspective
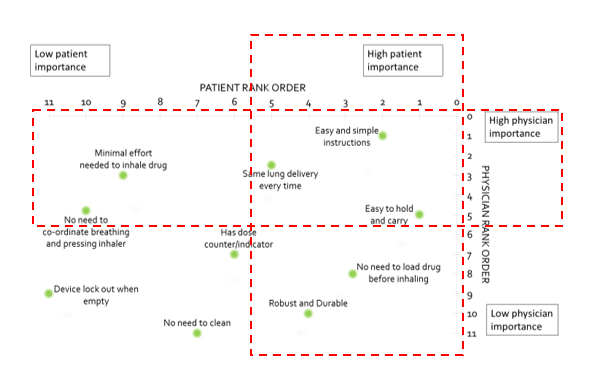
In a real-world study evaluating the preferences of patients and physicians regarding various aspects of inhaler design (Figure 2), “easy and simple instructions”, ”easy to hold and carry” and “same lung delivery every time” were considered parameters of high importance common to both patients and physicians. Besides these, other parameters important to physicians were “minimal effort to inhale drug” and “no need for coordination between actuation and inhalation” while, for patients, the other important parameters were “robust and durable” and “no need to load drug before inhaling”.
Understanding Physician and Patient Perceptions of Available Inhalation Devices in India: The INSPECT Survey
The INhalation deviceS: Physicians’ PErCepTions (INSPECT) survey was a cross-sectional questionnaire-based survey conducted by Cipla Ltd. to understand physician (n=83) and patient (n= 1,486) perceptions on currently available inhalation devices: pMDIs, pMDI+Spacer, DPIs, and breath-actuated inhalers (BAIs).
- More than 50% of patients desired a change in the current devices they used. “Fewer steps of use” and “simple and easy instructions to follow “ were the top two attributes in which the patients desired a change compared with their current device.
- Inability to use the inhaler device was the most common reason cited by doctors for poor control. Complexity of device design and the difficulty in using it were the factors thought to be responsible for reduced adherence and satisfaction with treatment.
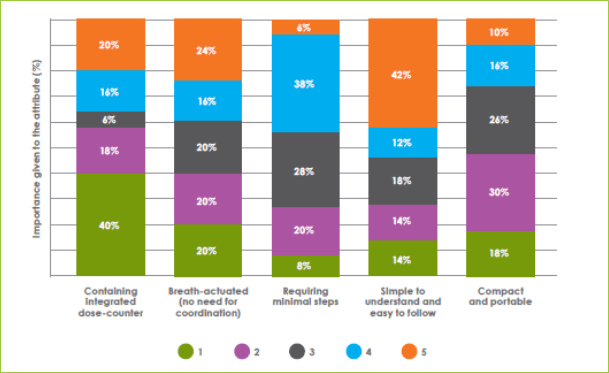
- · Physicians were asked to rate each of the attributes of inhalation devices on a scale of 1 to 5 (1 being least important and 5 being most important). “Simple to understand and easy to follow” was found to be the most important desirable attribute of an inhaler device, followed by “breath-actuated”, “integrated dose counter”, “compact and portable” and “minimal steps to use”.
Thus, the INSPECT study highlights the importance of developing devices that are simple to understand and use, require few steps, are breath-actuated, and are cost-effective.
The issues encountered with the existing inhaler devices have led to the development of BAIs with the aim of combining the beneficial features of pMDIs and DPIs.
Breath Actuated Inhalers
BAIs sense an inhalation effort through the actuator and mechanically actuate the dose in synchrony, resolving the issue of patient coordination of actuation with inhalation. Thus, BAIs overcome the drawback associated with lack of co-ordination and inability of patients to synchronize actuation with inhalation.
Bridging the Gaps
BAIs fulfil most of the criteria with respect to desirable features in a device.
Greater Ease of Use
- Fergusson et al. showed that 97% (n=151) of patients (n=156) with severe airflow limitation could actuate a BAI on their first (n=146) or second (n=5) attempt.[8]
- In a study done to evaluate patients’ perceptions (n=42) of BAIs, 98% found that the BAIs were easy to use.[9],13
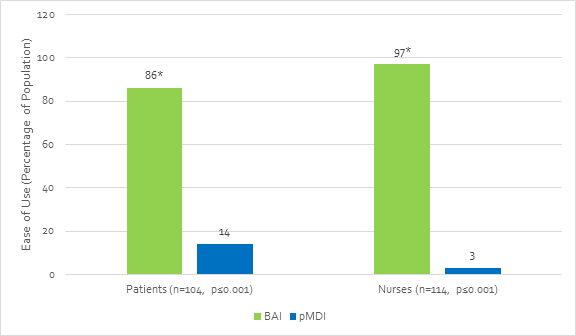
- Another study by Kelloway et al. demonstrated that significantly more patients and nurses found that the BAI was easier to use correctly compared with a conventional pMDI (Figure 4, p≤0.001). Nurses also believed that the BAI was easier to teach and use correctly in an acute attack (p≤0.001) compared with the pMDI.[10]
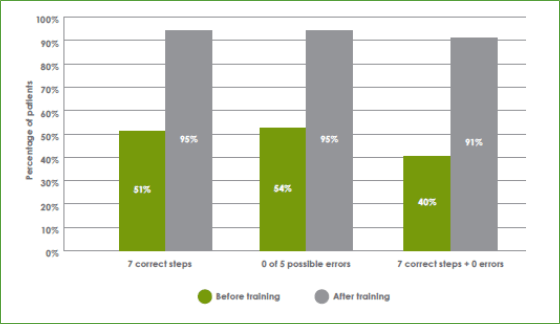
- That breath-actuated devices are easier to teach and use was further confirmed in the Sirocco study in which approximately 91% of patients with asthma (n = 6,512) could complete all procedural steps required to correctly use a breath-actuated device without error after a training session of approximately 4 minutes (Figure 5).11
- The ease of use of BAIs translates potentially into improved adherence, compliance and persistence with the prescribed therapy, and thereby improved disease control.12
Higher Lung Deposition
- Terzano et al. reported greater lung deposition of inhaled salbutamol delivered in-vitro by a BAI (~61%) compared with a pMDI (48%).13
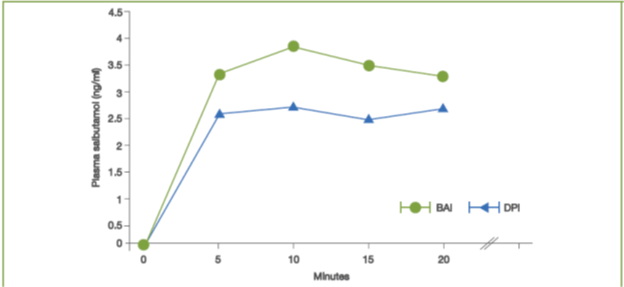
- In another study, the lung deposition of salbutamol was greater with a BAI as compared with a DPI, when assessed by measuring peak salbutamol concentrations (Cmax) at 5, 10, 15 and 20 minutes post-dose and average salbutamol concentration over 20 minutes post-dose (Cav) (Figure 6).14
Improved Lung Function
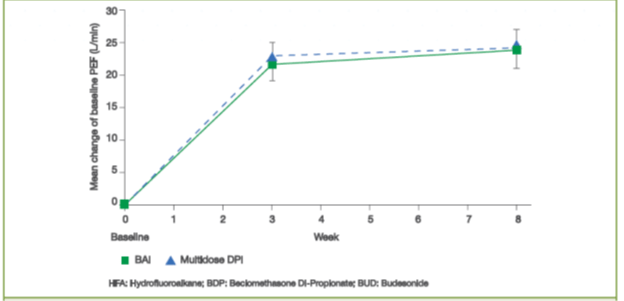
- The mean change from baseline in morning Peak Expiratory Flow rate (PEF) after 3 and 8 weeks of treatment with an inhaled corticosteroid (ICS) delivered via a BAI was equivalent with that of ICS delivered via a DPI (Figure 7).15
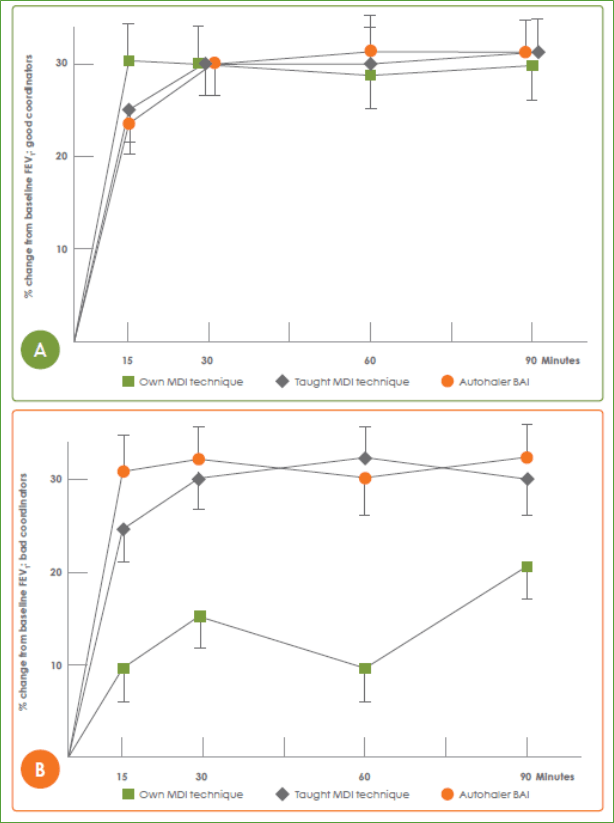
- In another study that assessed lung deposition of radiolabelled salbutamol in patients (n=19), each patient performed three radioaerosol studies on three different days separated by at least 48 hours. On day 1, patients used a radio-labelled pMDI with their ‘own pMDI technique’, while on day 2 and day 3, the patients were randomized to ‘taught pMDI technique’ and ‘BAI’, respectively.
o Lung deposition was shown to increase three-fold (21 vs 7%) with a BAI compared with a pMDI in subjects with poor coordination technique [own pMDI technique (7.2%) vs pMDI post-training (22.8%) vs BAI (20.8%)].16
o Also, delivery of salbutamol via a BAI resulted in resulted in comparable improvements in forced expiratory volume in first second (FEV1) in good and poor pMDI coordinators (Figure 8).
Correct Inhaler Technique and Greater Patient Preference with BAIs
Molimard et al. showed that critical errors (errors compromising treatment efficacy) in device use and inhalation technique (Figure 9) were made only by 11–12% of patients using the BAI (n=728) compared with 28% of patients using a pMDI (n=552).17
- Another study reported that more patients (n=104) preferred the BAI compared with a pMDI (82 vs 18%; p≤0.001). Ease of use and confidence in effective dose delivery were the key reasons for their preference. 18
- Steier et al. reported that a higher percentage of patients (n=200) with COPD preferred the BAI (62%) over the DPI (26%).18
Better Asthma Control
- Molimard et al. reported significantly greater improvements in asthma control [as assessed by asthma control questionnaire (ACQ) scores] with a BAI compared with multidose DPI (n=298, –1.0 ± 1.0 vs –0.6 ± 0.9; p=0.019).19
- The SYSTER survey20 assessed the influence of starting or switching an existing therapy to a breath-actuated pMDI (BA-pMDI). The study included 1,510 asthmatic patients in whom therapy with the BAI was either initiated, or a switch from an existing inhalation device to BAI was deemed necessary. Asthma control was assessed after 4 weeks of treatment.
o A significant improvement in asthma control and compliance was reported after 1 month on the BAI, with the mean ACQ score decreasing from 2.35 ± 1.05 to 1.32 ± 0.93 (p <0.0001).
o The proportion of patients with an ACQ score <2 showed an improvement by 42%. The self-reported patient adherence Morisky scale scores also improved from 2.11 ± 1.43 to 1.57 ± 1.53 (p<0.0001). (The Morisky scale is a self-reported medication-taking score based on four questions about forgetting the medicine, being careless at times about taking it, stopping it when feeling better or worse.)
Useful in Elderly Patients
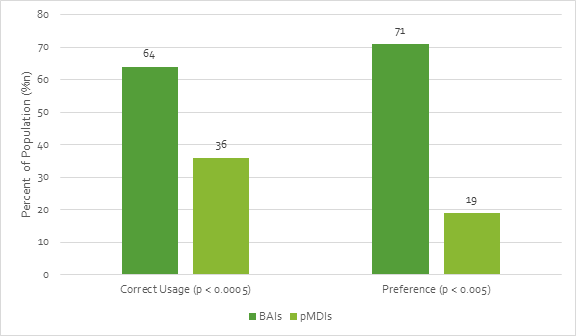
- Chapman et al. reported that elderly patients (n= 80) used BAIs correctly 64% of the time as compared with only 36% of the time with a pMDI.[21] The study further reported that a significant percentage of patients preferred BAIs compared with conventional pMDIs (Figure 10).
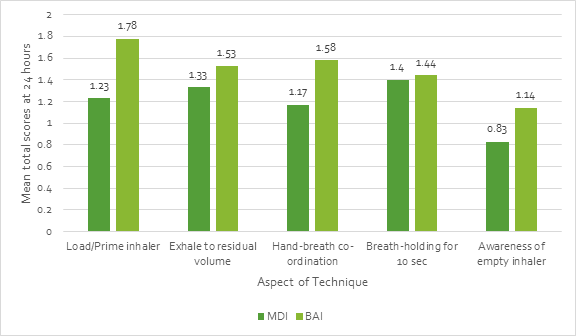
- A study by Jones et al. involving 102 inhaler-naïve elderly patients (mean age: 84 years) reported that BAIs were more likely to be used correctly than pMDI/ spacers.22
o Mean total scores for correct use of an inhaler device were significantly (p<0.005) higher for the BAI as compared with the pMDI.
o Mean total scores were significantly (P<0.005) higher for multidose DPI and BAI than pMDI/spacer on enrolment and at 6 hours and at 24 hours (P<0.045).
o The main difficulties were in assembling the pMDI/ spacer and detecting when the pMDI/spacer or BAI was empty (Figure 11).
Introducing Synchrobreathe® : A Novel Breath-Actuated Inhaler
Synchrobreathe® is a novel BAI with a built-in dose counter. The design of the Synchrobreathe® device incorporates the compactness, portability and ease of use of pMDIs with the ‘actuation on inspiration’ feature of DPIs.
Design Aspects
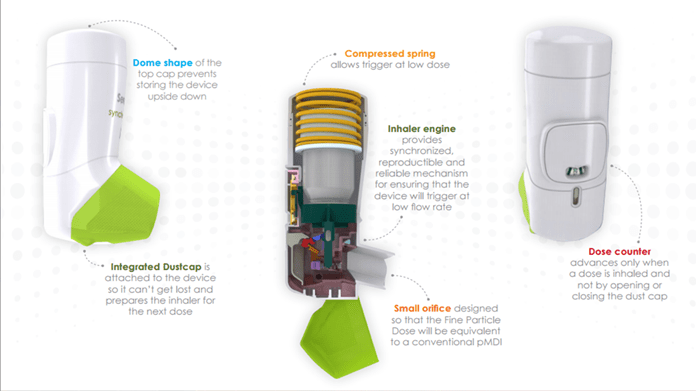
The Synchrobreathe® inhaler is a state-of-the-art innovation from Cipla. It comprises 23 parts that work in synchronization to deliver a standard, uniform dose with just a simple inhalation by the patient.
Features
|
PART |
WHY IS IT IMPORTANT? |
|
Integral Dust Cap |
Primes device and ensures that dust cap cannot get lost. Allows inhaler to remain free from ingress of foreign bodies. |
|
Orifice |
Small size ensures that fine particle dose is equivalent to pMDI. |
|
Engine Housing |
Can be amended to ensure that the device is suitable for all valve types. |
|
Device Engine |
Provides a reproducible and reliable mechanism for ensuring that the device will trigger at a low flow rate. Inhalation causes the flap to move and this causes a small trip link to pivot, which, consequently, means that the can lowers and fires. |
|
Dose Counter |
Counts each dose and moves every 20 puffs. When 40 puffs are left, the colour on the dose counter ring changes from green to red, indicating that the patient should organize for a new device. The dose counter does not lock out at 0 but the device should no longer be used when the counter reaches 0. |
|
Canister |
Identical to the pMDI canister and valve. |
|
Container Holder |
The legs of this component interact with the dust cap to prime the device, i.e. to leave the spring back up after a dose has been released. If the patients were to inhale again without closing the dust cap, then a further dose will not be released. |
|
Biasing Spring |
This is held under compression until the dust cap has been opened and the patient inhales at a flow rate that triggers the movement of the flap. The collapsing of the engine mechanism then causes this spring to ‘force’ the can downward to fire a dose. |
|
Top Cap |
Dome-shaped design has been selected to ensure that patients do not store the device upside down. |
Mechanism of Operation
The biasing spring is held under compression until the dust cap has been opened and the patient inhales at a flow rate that triggers the movement of the flap. The collapsing of the engine mechanism then causes this spring to ‘force’ the can downward to fire a dose.
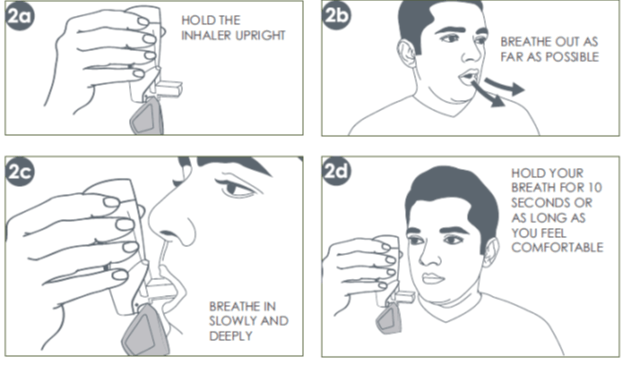
How to Use Synchrobreathe®
The Synchrobreathe® is a device that is very simple to use. Essentially, there are only three steps involved for one puff, namely, SHAKE & OPEN, BREATHE and CLOSE, as enumerated below.
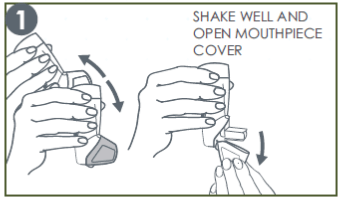
1. Shake & Open
Shake the Synchrobreathe® and open. (Prime the inhaler if using for the first time or unused for a week or more. Refer to the patient information leaflet for specific instructions on testing the Synchrobreathe®.)
2. Breathe
A: Hold the Synchrobreathe® in an upright position.
B: Breath out normally as far as comfortable (to empty the lungs).
C: Place the mouthpiece of the Synchrobreathe® in the mouth and close the lips around it. Start breathing slowly and deeply through the mouthpiece. Do not stop breathing on hearing a click and whoosh sound. It is important to keep breathing in after the puff is released. Maintain a slow and deep inhalation, through the mouth, until the lungs are full of air.
D: At the end of the inhalation, take the inhaler out of the mouth and close the lips. Continue to hold the breath for 10 seconds, or as long as possible.
3. Close
For the next puff, repeat steps 1 to 3. After taking the dose, rinse mouth well with water and spit out. Do not swallow. Always close the mouthpiece cover immediately after every puff. This prepares the Synchrobreathe® for the next use.
Cleaning of the Synchrobreathe®
Like all devices it is important to clean the Synchrobreathe periodically. Cleaning of the mouthpiece is to be done by wiping with a clean dry cloth or tissue and is recommended once a week.
Patients should not push any cloth or anything else into any part of Synchrobreathe as this may damage its operating parts. It is important to note that Synchrobreathe should NEVER be washed. It should always be kept dry.
Composition
SEROFLO Synchrobreathe® 125
Each actuation delivers:
Salmeterol…………………………....25 mcg
Fluticasone Propionate …………..125 mcg
SEROFLO Synchrobreathe® 250
Each actuation delivers:
Salmeterol…………………………....25 mcg
Fluticasone Propionate ……………250 mcg
Dosage and Administration
Asthma (aged 12 years and older)
SEROFLO Synchrobreathe® 125: Two inhalations twice daily
SEROFLO Synchrobreathe® 250: Two inhalations twice daily
COPD
SEROFLO Synchrobreathe® 125: Two inhalations twice daily
SEROFLO Synchrobreathe® 250: Two inhalations twice daily
For details, refer to the patient information leaflet inside the package of Synchrobreathe®.
Synchrobreathe®: The Evidence
In vitro Lung Deposition
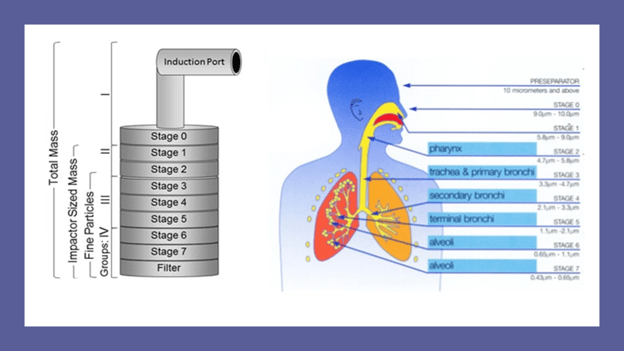
Fine particle mass (FPM) is the amount of drug less than 5 microns of aerodynamic diameter and gives an indirect estimation of the amount of drug delivered to the lungs.
To assess the FPM of Synchrobreathe® vs pMDI, doses of SEROFLO Synchrobreathe® 250 and SEROFLO pMDI 250 were aerosolized into a cascade impactor at a flow rate of 45 L/min.
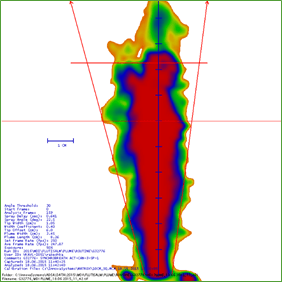
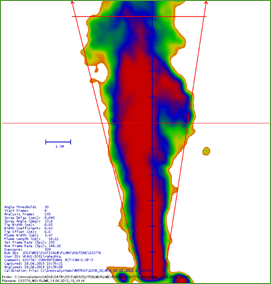
The cascade mimics the human respiratory tree and has a vertical planar layout, which has been adapted for ease of operation and automation. It has seven stages, five of which are in the range of 0.5 to 5 microns, plus a micro-orifice collector, which acts as a final filter (Figure 13).
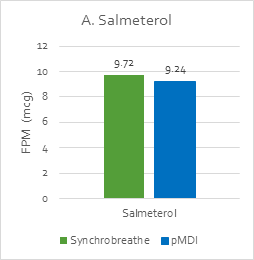
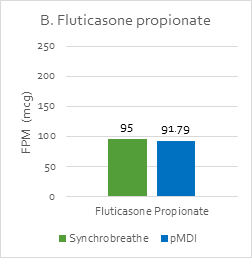
The FPM with Synchrobreathe® was similar to that with conventional pMDI for both salmeterol and fluticasone (Figure 14).
Synchrobreathe® inhaler is designed to be triggered with a very low inspiratory flow rate ranging from approximately 23 to 35 L/min. Even young patients and patients with severe lung conditions should be able to use the inhaler.
In-vivo Study
A prospective, randomized, open-label, cross-over, two-period, single-dose study aimed to evaluate lung deposition of salmeterol and fluticasone propionate (25 mcg /125 mcg) when delivered from the Synchrobreathe® device compared with the conventional pMDI in patients with stable persistent asthma.
Subjects of either gender between 18 and 70 years (both inclusive) of age with physician-diagnosed asthma according to GINA 2015 guidelines, who were on SEROFLO pMDI (25/125 in one puff) and had poor coordination of actuation with inspiration were included.
Ten stable adult patients with asthma received one puff of radiolabelled (with Technetium-99m) SFC (25/125mcg) via
either the Synchrobreathe® or a conventional pMDI (both Cipla Ltd, India) on the first treatment day,
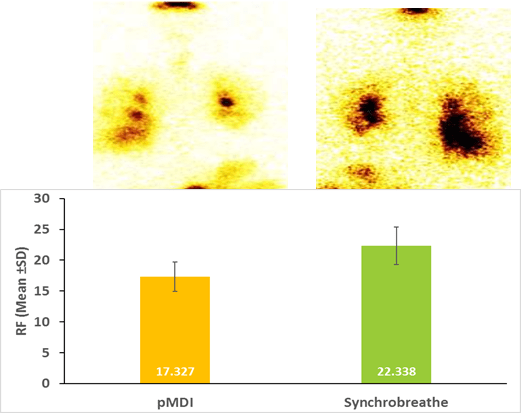
and via the alternative device on the second treatment day. The study results demonstrated a greater lung deposition
with Synchrobreathe® compared with the pMDI device (Figure 15).
Consistency of Dosing
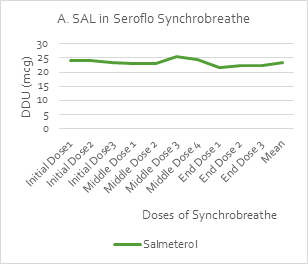
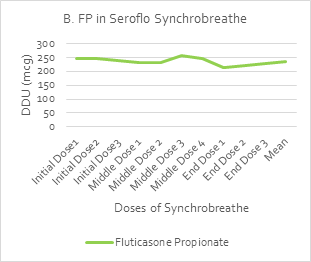
An in-vitro dose delivery performance test [by UDD Apparatus (Copley Scientific)] was used to measure the uniformity of the delivered dose (UDD) over the initial, middle and end doses of Synchrobreathe® inhaler for salmeterol (SAL) and fluticasone propionate (FP). A steady dose was delivered throughout the 120 doses of the test samples, as shown in Figure 16.
Spray Pattern and Plume Geometry
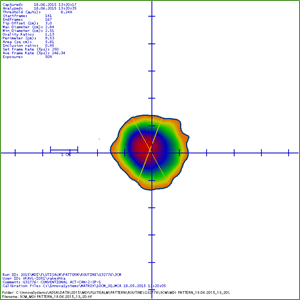
A study was conducted to identify the spray pattern and particle size to gain a more complete understanding of spray plume measurements for Synchrobreathe® and conventional pMDI by ADSA (Aerosol Drug Spray Analyzer). The pattern and plume geometry measurements were recorded as shown in Figure 17.
Synchrobreathe® Conventional pMDI
|
Spray Pattern |
||||
|
Actuator: |
Conventional pMDI actuator (0.48 mm) |
SYNCHROBREATHE® |
||
|
|
Ovality ratio |
Area |
Ovality ratio |
Area |
|
Average Value |
1.17 |
5.82 |
1.20 |
5.73 |
|
Plume Geometry |
||||
|
|
Spray angle |
Plume width (cm) |
Spray angle |
Plume width (cm) |
|
Average Value |
23.71 |
3.66 |
23.62 |
3.53 |
The Synchrobreathe® device was shown to produce a spray pattern, plume width and plume angle similar to that of a conventional pMDI (Table 3).
Robustness
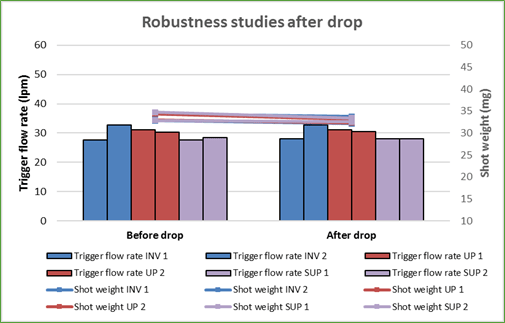
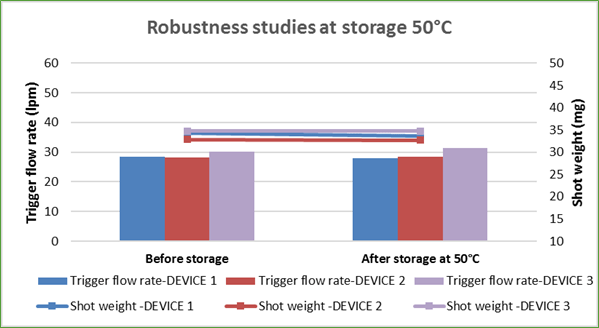
The Synchrobreathe® was tested for robustness by dropping the device from a height of 5 feet at various orientations – inverted, supine and upright, and was checked for trigger flow rate and shot weight. Trigger flow rate is the flow rate at which the inhaler triggers the release of the drug formulation. Shot weight is the average weight of formulation per metered dose. The test results showed that there was no change in the trigger flow rate and the shot weight of the puff released (Figure 18).
The Synchrobreathe® was also tested for robustness by storing it at 50°C and room humidity 75% and was checked for trigger flow rate and shot weight (Figure 19). Again, there was no change in the trigger flow rate and the shot weight of the puff released (Figure 19).
This shows that the Synchrobreathe® is a robust device.
Device-Handling Study
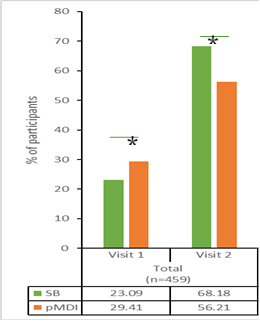
An open-label, prospective, comparative, multicentre study involving 460 subjects (healthy volunteers, 59; asthma, 239; COPD, 162)] was designed to evaluate device handling, human factors, ease of use, errors and participant perception with Synchrobreathe® inhaler vs pMDI. Current or past users of BAIs were excluded. Data was captured in the study on day 0 (visit 1) and the end of treatment period at day 14 (visit 2).
The results showed that the percentage of participants who could use both the inhaler devices without errors after reading the patient instruction leaflet only (*p<0.05; **p<0.01; ***p<0.001 versus pMDI) was more with the Synchrobreathe® at visit 2 (day 14) when compared with the pMDI (Figure 20)
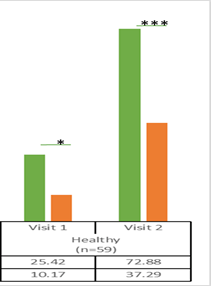
The percentage of healthy participants who could use the inhaler device without errors after reading the patient instruction leaflet only at both visits was also significantly higher with the Synchrobreathe® when compared with the pMDI (Figure 21).
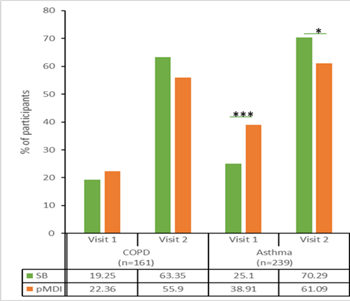
Also, the percentage of COPD and asthma participants who used both the inhaler devices without errors after reading the patient instruction leaflet only were higher with Synchrobreathe® at visit 2 (Figure 22) than with the pMDI.
Importantly, the average time (in seconds) required to perform the inhalation technique correctly during the first and second visit for Synchrobreathe® was lesser than that for pMDI and the difference between visits 1 and 2 were all significant (p<0.0001).
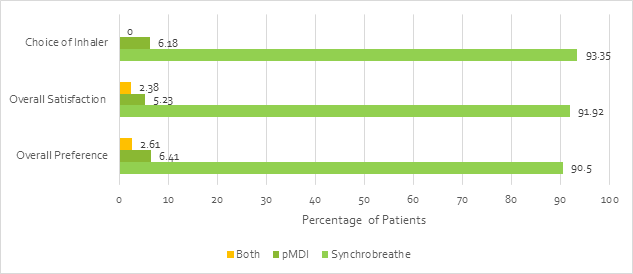
Overall, the patient feedback (n=421) showed that the Synchrobreathe® was preferred over the pMDI by a greater percentage of patients (Figure 23).
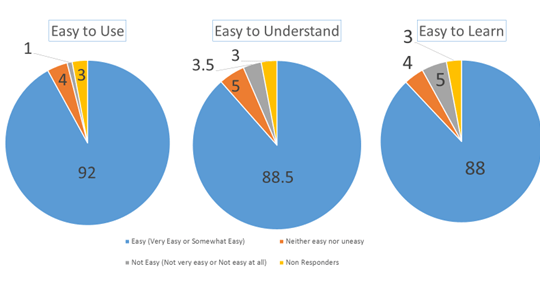
The study also showed that Synchrobreathe® was easy to use. Overall, 92% of patients found “Synchrobreathe® an easy-to-use device”, 88.5% of patients said “Steps of Synchrobreathe® are easy to understand”, and 88% of patients opined that “Synchrobreathe® was easy to learn” (Figure 24).
The study concluded that Synchrobreathe® is easier to learn and teach when compared with the pMDI. Overall, Synchrobreathe® was the preferred device and enjoyed a higher level of patient satisfaction.
Summary
Synchrobreathe® is a state-of-the-art BAI researched, developed in-house and patented by Cipla to help patients achieve better control of their airway disease. Synchrobreathe® is easy for healthcare providers to demonstrate and teach, while for the patients, it is easy to learn and remember to use correctly.
|
Features |
Benefits |
|
3 intuitive steps |
Easy for physicians to teach and patients to learn and remember |
|
Overcomes synchronization issues |
No press and breathe coordination is required |
|
Operates optimally even with low inspiratory flow |
Ensures it will be easy for patients to use, regardless of age or disease severity |
|
Contains dose counter |
Dose counter counts every puff and might improve compliance |
|
Contains the most widely inhaled respiratory combination globally: Salmeterol and Fluticasone propionate |
Trusted by healthcare providers and patients alike |
Additionally, the Synchrobreathe® device requires a low inspiratory flow rate for breath-actuation, which means that young patients, elderly patients and patients with severe airflow obstruction will be able to use the device and be assured of a consistent dose every time they use it. The Synchrobreathe® device also has an integrated dose-counter that helps patients keep a track of the doses remaining in the device and this, in turn, can help in improving compliance.
Overall, Synchrobreathe® is a well-researched device and backed by evidence that establishes its superiority over pMDIs. It helps overcome the widely acknowledged problem of coordination between actuation and inhalation in pMDIs and results in a greater lung deposition of the medication vs a pMDI. The device is proven to be robust enough to deliver consistent medication over 120 doses. The device also appears to be a preferred device over pMDIs, with a higher level of patient satisfaction as it offers ease of usage, understanding and learning.
Synchrobreathe® truly represents a new era in managing airway diseases.
References
1. Chest 2016; 150(2):394-406.
2. Respir Care 2005;50(10):1360 –1374.
3. Eur Respir J 2002; 19, 246–251
4. Chronic Respiratory Disease 2017, Vol. 14(3) 309–320
5. Respir Med. 2008; 102, 4, 593–604
6. Eur Respir J. 2008; 31, 78–83
7. J Aerosol Med Pulm Drug Deliv. 2017, 30(1): 1-13
8. Eur Respir J 1991; 4, 172-174
9. J. Asthma 1993, 30, 439–443
10. J. Asthma 1993;30, 373–379
11. Eur Rev Med Pharmacol Sci. 2011;15(5):563-70
12. Expert Rev. Respir. Med. 2014; 8(1), 89–99
13. Monaldi Arch Chest Dis. 1996 Jun;51(3):236-42.
14. Pulm. Pharmacol. Ther. 1997. 10, 211–214
15. Eur Rev Med Pharmacol Sci. 2001. 5, 7-16
16. Thorax 1991; 46, 712–716
17. J Aerosol Med. 2003 Fall;16(3):249-54.
18. Lung. 2003;181(4):183-92
19. Respir Med. 2005 Jun;99(6):770-8
20. Eur Rev Med Pharmacol Sci. 2009 Sep-Oct;13(5):323-30
21. Chest. 1993 Nov;104(5):1332-7
22. Age Ageing. 1999 Sep;28(5):481-4