Chronic Obstructive Pulmonary Disease
Prevalence of Abnormal Spirometry in Ever Smokers with No Known Obstructive Lung Disease
Rationale: This study was aimed to determine the prevalence of abnormal spirometry in smokers who did not have a known obstructive lung disease in the COPDGene cohort.
Methods: 10,104 ever smokers in the COPDGene study were included who had available post-bronchodilator spirometry at the time of enrollment. 5,171 participants were excluded. All those with a physician diagnosis of asthma, chronic obstructive pulmonary disease (COPD), chronic bronchitis, emphysema, and/or receiving treatment for obstructive pulmonary disease (OPD) at the time of enrolment were excluded. Patients were divided into categories based on their spirometric pattern: i) normal (FEV1/FVC>0.8, FEV1>80%pred)), ii) preserved ratio impaired spirometry (PRISm; (FEV1/FVC>0.8, FEV1<80%pred)) and iii) airflow obstruction (AFO; (FEV1/FVC<0.8, FEV1<80%pred)). To identify factors associated with AFO or PRISm, the investigators created parsimonious multivariable logistic regression models. Clinically relevant variables were considered for multivariable analyses. Variables were selected for the final model using a stepwise backward variable elimination process to minimize the Akaike information criterion. Whether the participants self-reported an OPD diagnosis and/or treatment at the 5-year follow-up visit was assessed.
Results: 4,933 participants did not self-report any OPD diagnosis or treatment at the baseline visit. Of these, 3,236 (65.6%) had normal spirometry, 663 (13.4%) had PRISm, and 1,034 (21%) had AFO. It was found that compared to normal, participants with AFO were more likely to be older (OR for every 10 years=1.91;95%CI=1.72-2.12), current as opposed to former smokers (OR=2.17;95%CI=1.79-2.6), have a history of acute bronchitis (OR=1.39;95%CI=1.16-1.66), chronic productive cough (OR=1.31;95%CI=1.04-1.65), more pack years (OR for every 10 pack-year=1.15;95%CI=1.11-1.19) and a modified medical research council (mMRC) dyspnea score ≥2 (OR=1.52;95%CI=1.23-1.87) while less likely to be female (OR=0.78;95%CI=0.66 -0.91) and black (OR=0.56;95%CI=0.46-0.68) or have a lower BMI (OR for every 1 unit=0.96;95%CI=0.95-0.98). Similar findings were observed among those with PRISm except that it was found that higher BMI was a risk factor for PRISm. Approximately, one-quarter to one-third of individuals with AFO and PRISm had a history of prior acute bronchitis or pneumonia at enrollment. One-third of individuals with AFO and one-quarter of individuals with PRISm carried an OPD diagnosis and/or received treatment at the 5-year follow-up visit.
Conclusions: Approximately one-third of ever smokers who were never diagnosed with OPD and had never received any treatment for OPD had an abnormal spirometry, with nearly two-thirds of those patients having AFO in the COPDGene cohort. Those who had a respiratory event prior to the enrollment when an OPD diagnosis could have been considered was approximately, one-quarter to one-third of these individuals
A2213; Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.
Wearable Sensors for Early Detection of COPD Exacerbations
Rationale: Few automated real-time algorithms for early detection of an acute exacerbation of COPD are available. Early treatment of these acute exacerbations can lead to improved outcomes. This study hypothesized that real-time sensors combined with an eDiary could be used before healthcare utilization (HCU) in order to detect exacerbations.
Methods: All those COPD patients who had atleast one exacerbation within the previous 12 months of enrollment (n=19; data collection ongoing) & provided informed written consent for a year-long data collection with FitBit (hourly steps, heart rate), Propeller (daily rescue inhaler), and EXACT (daily eDiary) were included. A pulmonologist reviewed the Medical records and adjudicated for exacerbations (requiring treatment with corticosteroids and/or antibiotics). Starting 7 days prior to the first HCU and ending the day of the HCU Sensor and eDiary data were assessed in windows. These windows were compared to 7-day baseline windows ending 14 days before the HCU (Figure 1). For heart rate data, a t-test was used for comparison between baseline vs pre-exacerbation means. The Mann-Whitney U test was used for step and inhaler puff counts, and EXACT score.
Results: Subjects were followed up for a median time period of 11 months (IQR=2 months). In total, there were 8 adjudicated exacerbations from 4 of the 19 subjects. 4 (57.1%) showed significant differences between the baseline and pre-exacerbation window means of the 7 exacerbations with available heart rate data. 1 (14.2%) showed a significant difference relative to baseline Of the 7 exacerbations with step count data. 3 (42.8%) showed significant differences in EXACT score relative to baseline mean Of the 7 exacerbations with available EXACT data. Only 1 exacerbation was an EXACT-defined event. For the 6 events with available inhaler data, 2 (33%) showed significant differences in daily puff count relative to baseline. Overall, 7 of the 8 (87.5%) exacerbations showed a significant difference in at least one data source.
Conclusions: The use of eDiaries & sensors was found to have a long term compliance amongst most subjects. Exacerbations had heterogeneous sensor abnormality patterns and most AECOPDs had at least one observable change in sensors before their HCU. This justifies the use of multiple sensor streams. This could allow healthcare providers to intervene earlier in AECOPD events, thus reducing exacerbation severity and preventing hospital admissions.
A1300; Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.
A Post-Hoc Analysis of the Effect of Smoking Status on COPD Exacerbation Reductions with Budesonide/Glycopyrrolate/Formoterol Fumarate in Patients with COPD in the ETHOS Study
Rationale: Cigarette smoking is a leading cause of chronic obstructive pulmonary disease (COPD). In the 52-week ETHOS study (NCT02465567), fixed-dose inhaled corticosteroid (ICS)/long-acting muscarinic antagonist (LAMA)/long acting β2-agonist (LABA) triple therapy with budesonide/glycopyrrolate/formoterol fumarate (BGF) reduced the rate of moderate or severe COPD exacerbations versus fixed-dose dual therapy with the LAMA/LABA glycopyrrolate/formoterol fumarate (GFF) or the ICS/LABA budesonide/formoterol fumarate (BFF) in patients with moderate-to-very severe COPD. Current smokers have a higher risk of COPD exacerbations, and, it has been reported, may derive less treatment benefit from ICS (Sonnex K, et al. BMJ Open 2020;10:e037509). This post-hoc analysis explored the effects of BGF versus GFF and BFF on exacerbations of COPD in current and former smokers in the ETHOS study.
Methods: ETHOS was a multinational, randomized, double-blind, parallel-group study conducted in patients with moderate-to-very severe COPD who experienced ≥1 moderate or severe exacerbation in the previous year. Patients were randomized 1:1:1:1 to receive twice-daily treatment with BGF 320/18/9.6 µg (BGF 320), BGF 160/18/9.6 μg, GFF 18/9.6 µg, or BFF 320/9.6 µg via a single metered dose Aerosphere inhaler. All patients had a smoking history of ≥10 pack-years. The current post-hoc analysis assessed annual rates of moderate or severe exacerbations and annual rates of severe exacerbations in subgroups of patients defined by smoking status at randomization using the modified intention-to-treat (mITT) population, which was defined as all randomized patients who received treatment and who had post-randomization data.
Results: Of 8509 patients included in the mITT population, 3495 were current smokers and 5014 were former smokers. BGF 320 reduced the annual rate of moderate or severe exacerbations versus dual therapy with GFF or BFF, regardless of smoking status (Table). The annual rate of severe exacerbations was also lower with BGF 320 versus dual therapies, although there were few events in these subgroups (Table).
Conclusions: Fixed-dose ICS/LAMA/LABA triple therapy with BGF 320 reduced the annual rate of moderate or severe COPD exacerbations versus fixed-dose dual therapy with either LAMA/LABA or ICS/LABA in patients with moderate-to-very severe COPD regardless of smoking status.
A1451; Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.
Risk for Exacerbation, Hospitalization and Mortality in Global Initiative for Chronic Obstructive Lung Disease Group B Patients With and Without Exacerbations: A Cohort Study
Rationale: It is clinically important to stratify COPD patients according to severity & it forms the basis of therapeutic recommendations. There are no previous studies which have examined the association for GOLD group B patients with (B1) and without (B0) an exacerbation in the last year with future exacerbations, hospitalizations, and mortality.
Methods: In this nationwide cohort study, patients with a primary diagnosis of COPD, aged 30 years or older who had been registered at least once in the Swedish National Airway Register (SNAR) from January 2017 to August 2020 were enrolled. Based on COPD Assessment Test (CAT) score and/or mMRC-dyspnea score on index date (first visit with available CAT/mMRC) and exacerbation/hospitalization history in the year before index date patients were stratified in groups A, B0, B1, C and D. They were followed from index date until January 2021 for exacerbations, hospitalization and survival. Date of death was obtained from the National Cause of Death Register, hospitalization date from the National Patient Register and date of non-hospitalized exacerbation was defined as the dispension date of a course of oral corticosteroids from the Swedish Prescribed Drug Register.
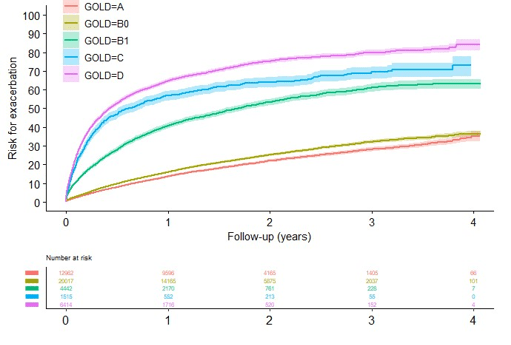
Results: 70844 patients registered were registered. Of these, in total (64%) 45350 had available CAT or mMRC and were eligible for the current study. 29% had GOLD A, 44% GOLD B0, 10% GOLD B1, 3% GOLD C and 14% GOLD D. Cumulative risk for a first exacerbation, all-cause and respiratory cumulative hospitalization risk as well as all-cause and respiratory cumulative mortality increased with increasing symptoms and exacerbations, i.e. increasing GOLD group. Exacerbation risk was similar in group B0 and higher in group B1, closer to group C (Figure 1) compared to GOLD group A. There was a significant and substantial higher risk of future exacerbation (HR 2,72 (95%CI: 2,59-2,87), all-cause hospitalization (HR 1,24 (95%CI: 1,18-1,31), respiratory hospitalizations (HR: 1,69 (95% CI: 1,55-1,85), all-cause mortality (HR: 1,19 (95%CI: 1,08-1,31) and respiratory mortality (HR: 1,62 (95%CI: 1,26-2,07) in GOLD Group B1 compared to group B0. As compared to group B1, the exacerbation rate for group B0 was 0.2 events per patient-years versus 0.6 (RR: 2,73 (95% CI: 2,57-2,79).
Conclusion: Valuable information on future risk is obtained from stratification of GOLD B patients for one or no exacerbation in the last year provides. This should influence treatment recommendations to formulate effective preventive strategies.
Figure 1: Cumulative risk for a first exacerbation in groups A, B0, B1, C and D
A5530; Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.
Effect of Peak Inspiratory Flow Data on Physician Prescribing Practices of Dry Powder Inhalers for COPD Patients
Rationale – In clinical practice Peak inspiratory flow (PIF) measurement can help in assessing whether a patient has the inhalation ability to generate an optimal PIF for use with a dry powder inhaler (DPI). This study was aimed to review the characteristics of COPD patients on DPI inhalers and also to assess whether physician prescribing was affected by informing providers of PIF measurements.
Methods – This was a observational, prospective quality improvement study which was conducted among 48 patients with COPD treated with a DPI seen at NorthShore University HealthSystem pulmonary outpatient clinics. The data collected included demographics, medication usage, smoking history, spirometry, GOLD grouping, mMRC score, and prior exacerbation history via chart review. PIF measurements against simulated DPI resistance using the In-Check DIAL™ was collected. Suboptimal PIF was defined as <60 L/min for patient’s using DPI with medium-low resistance (Diskus and Ellipta), <45 L/min for medium resistance (Pressair), < 30 L/min for high resistance (Handihaler). The PIF data was presented to the managing pulmonologist and their response in terms of inhaler prescription was recorded.
Results – 48 patients were studied with mean age of 75.8 years. Amongst these 62.5 % were female. Mean mMRC was 1.78, mean % predicted FEV1 was 54.0%. 68.75% of all patients had suboptimal PIF for the inhaler previously prescribed. In our data set suboptimal PIF was not significantly correlated with age, gender, height, pack years of smoking, % predicted FEV1, or number of exacerbations in the past year. Pulmonologist elected to prescribe a different inhaler for 12% of patients with a suboptimal PIF compared to 7% of patients with an optimal PIF, although this was not a significant difference.
Conclusions - The majority of patients had suboptimal PIF. Suboptimal PIF rates were seen in patients of all ages, gender, smoking history, and COPD severity. It rarely led to a change in prescribing decision and no significant difference was seen between optimal versus suboptimal PIF levels and physician prescribing patterns. Despite calls for physicians to consider PIF in their choice of inhaler for COPD patients, in our study that rarely occurred in clinical practice despite providing PIF data to prescribers. Further studies are needed to determine the clinical implications of suboptimal PIF on patient outcomes, physician knowledge and practice patterns in relationship to PIF data and prescription decision-making.
A2786; Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.
Comparative Efficacy of Fluticasone Furoate/Umeclidinium/Vilanterol (FF/UMEC/VI) Versus Other Triple Therapies for the Treatment of Chronic Obstructive Pulmonary Disease (COPD): A Systematic Literature Review and Network Meta-Analysis
Objective: The objective of the network meta-analysis (NMA) was to investigate the efficacy of fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) compared to other triple therapies, including single inhaler and multiple inhaler therapies, in COPD (chronic obstructive pulmonary disease) patients.
Methods: Based on a systematic literature review (SLR), NMA in a Frequentist framework was conducted. RCTs were identified by the SLR in adults aged ≥40 years with COPD that compared ICS/LABA/LAMA combinations versus other treatment regimens. Mean differences in change from baseline (CFB) at weeks 12, 24 and 52 in trough forced expiratory volume in 1 s (FEV1) were determined. Both fixed effect (FE) and random effects (RE) models were used in this NMA. To examine the robustness of the data to the inclusion of open-label studies, sensitivity analyses were conducted.
Results: 23 studies were identified by the SLR reporting data on trough FEV1 at 12 or 24 weeks, but not all studies could be connected to the networks of evidence. As a result, the NMA was informed by 15 studies reporting FEV1 at 12 weeks, and 5 studies at 24 weeks. FF/UMEC/VI was statistically more significant and effective at increasing trough FEV1 (based on change from baseline) at 12 weeks than all triple comparators arms apart from TIO (tiotropium) 18 + SAL/FP (salmeterol/fluticasone propionate) 50/250, TIO 18 + BDP/FOR (beclomethasone dipropionate/formoterol) 100/6, and UMEC+FF/VI. At 24 weeks, FF/UMEC/VI was also statistically more significant and effective at increasing trough FEV1 (based on CFB) than all triple comparator arms in the network apart from UMEC+FF/VI as mentioned in table. No comparisons of FF/UMEC/VI versus other triple therapies were feasible at 52 weeks.
Conclusions: The outcomes of this NMA suggest favourable long-term efficacy with single-inhaler triple therapy comprising FF/UMEC/VI. It showed significant and more effective improvement in trough FEV1 when compared with other therapies.
A2795; Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.
The Effect of Inhaled Corticosteroid Withdrawal on Inflammation and the Airway Microbiome in COPD: The INCOGNITO Trial
Objective: The aim was to understand whether ICS withdrawal would decrease airway bacterial load and modify the airway microbiome compared to continued ICS treatment in patients with non-eosinophilic COPD.
Methods: Stable patients with moderate to severe COPD currently treated with ICS and a blood eosinophil count <300cells/ul were enrolled. Patients were randomised to receive either Tiotropium and Olodaterol (ICS withdrawal) or Fluticasone Furoate/Vilanterol (ICS continuation) for 6 months. Sputum, oropharyngeal and nasopharyngeal swabs were taken at baseline, months 1,2,3 and 6 and during exacerbation. Bacterial load and microbiome characterisation were performed by 16S qPCR and 16S rRNA sequencing. Exacerbations classification is done as bacterial, viral, eosinophilic or “other” on the basis of PCR detection of bacteria and viruses and eosinophil counts. Inflammatory markers were evaluated in sputum.
Results: 80 COPD patients were enrolled with baseline characteristics, mean age 69.3, 51.2% male, 32.5% current smokers with mean CAT score of 20.6. 38 patients were randomised to Tio/Olo, 42 to FF/VI. There was no difference in exacerbation rate or FEV1 between both the arms. Post one month, 26.1% of patients in the ICS withdrawal arm experienced a >4-point improvement in SGRQ compared to 7.1% in the FF/VI group (p=0.02). Between the treatment arms, no differences in bacterial load were detected. The microbiome was heterogenous, with many sequences identified as Proteobacteria or Firmicutes. ICS withdrawal was associated with statistically significant increases in Streptococcus abundance (p=0.01) and a non-significant reduction in Haemophilus dominated microbiome profiles. Alpha diversity was decreased following ICS withdrawal (p=0.04) associated with a higher relative abundance of commensal taxa including Rothia, Prevotella and Veillonella. Similar results were seen in the oropharyngeal and nasopharyngeal microbiota. There was no significant increase in inflammatory markers in sputum following ICS withdrawal except for sputum IL-5 which was increased in the Tio/Olo treatment arm (p=0.04). 30 patients in the Tio/Olo group and 25 patients in the FF/VI group were studied at exacerbation. There was a higher frequency of bacterial and bacterial-viral co-infection exacerbations in the ICS group and a higher frequency of eosinophilic and unexplained exacerbations in the ICS withdrawal group.
Conclusion: ICS withdrawal did not change the airway bacterial load but did alter the composition of the microbiome. A reduction in more severe, bacterial associated exacerbations were observed as ICS withdrawal altered the exacerbation endotype.
A5567; Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.
The Use of Diaphragm Ultrasonography to Predict Outcomes in Patients with Acute COPD Exacerbation
Objective: The aim of the study is to establish whether diaphragm dysfunction, as measured through diaphragm ultrasonography (DU), is related with worse clinical outcomes in acute COPD patients with exacerbations (ACOPDE) admitted to the medical floors.
Methods: Prospective observational study at an academic tertiary hospital. Patients included in the study were admitted to the medical floors with ACOPDE not requiring invasive or non-invasive ventilation (NIV). The patients excluded from the study were patients with diaphragm palsy, neuromuscular disorders, chronic steroid use, and pregnancy. Within 48 hours from admission, DU was performed on the right side at the zone of apposition, in a semi-setting position. Diaphragm thickness was measured at end-inspiration and end-expiration during quiet breathing. An average of at least two measurements was used. Diaphragm thickening fraction (DTF) was calculated as diaphragm thickness at end of inspiration - diaphragm thickness end-expiration/diaphragm thickness end-expiration x 100%. The diaphragm thickening ratio (DR) was analyzed as the difference between end-inspiration and end-expiration thickness in mm. Diaphragm dysfunction was defined as a DTF of less than 30%. The primary outcome was the use of NIV during hospitalization, and the secondary outcome was the hospital length of stay.
Results: A diaphragm US was performed on 16 patients (6 males, 10 females) with a mean age of 64.9. Most of the patients did not have an earlier pulmonary function test at admission, and 4 out of 16 patients were on home oxygen. The average length of stay was 5.5 days, and the average diaphragm thickness at end-inspiration was 29 mm, while end-expiration was 19 mm. The mean DTF and DR were 57% and 10 mm, respectively. Two patients (12.5%) had diaphragm dysfunction with DTF less than 30%. The length of stay of patients with diaphragm dysfunction was higher as compared to those without (11.5 vs 5.5 days). None of the patients required NIV during their hospital stay.
Conclusion: In patients with ACOPDE, diaphragm dysfunction can be detected through DU. It is also related with increased length of stay. In patients who don’t require mechanical ventilation or NIV for ACOPDE, Diaphragm dysfunction is infrequent. To reduce the length of stay, serial DU measurements might help change the care approach of these patients.
A1081; Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.
Asthma & Inhalation Devices
Evaluating the Effectiveness and Safety of Switching from Salmeterol - Fluticasone Inhalation to Formoterol - Budesonide Inhalation as Single Maintenance and Reliever Therapy (SMART) Approach in Partly Controlled or Uncontrolled Asthma Patients
Rationale: The current Global Initiative for Asthma (GINA) recommendations lays emphasis on single inhaler maintenance and reliever therapy (SMART). The current study assessed the effectiveness and safety of SMART therapy with the Formoterol–Budesonide (FB) combination in asthmatic patients who are partially or completely uncontrolled on Salmeterol-Fluticasone (SF) therapy.
Methods: Medical records from 24 sites across India were retrospectively evaluated for the change in Asthma Control Questionnaire-5 (ACQ-5) score. Secondary objectives included evaluation of change in mean pre-dose FEV1, mean FVC and in mean FEV1/FVC following switching to FB (SMART method) at 4 weeks and 8 weeks.
Results: Medical record of 122 patients were analysed. Average age of the recruited patients was 47.49 ± 11.26 years. The median duration of time since asthma diagnosis was 7.04 years. The mean ACQ-5 score decreased significantly (p<0.0001) by -1.48 at 4 weeks (3.59 ± 1.12 to 2.11 ± 0.99) and -2.47 at 8 weeks (3.59 ± 1.12 to 1.12 ± 0.73) after switching to SMART therapy. The pre-dose morning FEV1 (ml) did not improve significantly at week 4 (p=0.16) or week 8 (p=0.40). At the eighth week, FVC improved significantly (p=0.002). There was a significant improvement seen in FEV1/FVC at week 4 (p=0.01) and week 8 (p=0.0001). Only one adverse event (dysphonia) was reported.
Conclusions: Switching to FB SMART strategy improved symptom control and lung function at 4 and 8 weeks without giving rise to any critical safety concerns in patients who were partially or completely uncontrolled on SF treatment. Therefore, asthmatic patients who are partially controlled or uncontrolled on conventional inhaled corticosteroids and long acting beta agonist (ICS-LABA) combination can be switched to SMART strategy for improvement in asthma control.
A1315; Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.
Albuterol–Budesonide Fixed-Dose Combination Rescue Inhaler for Asthma: MANDALA Study
Alberto Papi, M.D., Bradley E. Chipps, M.D., Richard Beasley, D.Sc., Reynold A. Panettieri, Jr., M.D., Elliot Israel, M.D., Mark Cooper, M.Sc.
Background: Patients typically rely on short-acting β2-agonist (SABA) rescue therapy during worsening of asthma symptoms, but SABAs do not address worsening inflammation, which leaves patients at risk for severe asthma exacerbations.
Methods: A multinational, phase 3, double-blind, randomized, event-driven trial was conducted to evaluate the efficacy and safety of albuterol–budesonide vs albuterol alone, as rescue medication in patients with uncontrolled moderate-to-severe asthma who were receiving inhaled glucocorticoid-containing maintenance therapies. The primary efficacy end point was the first event of severe asthma exacerbation in a time-to-event analysis, which was performed in the intention-to-treat population. Adults and adolescents (≥12 years of age) were randomly assigned in a 1:1:1 ratio to one of three trial groups: a fixed-dose combination of 180 μg of albuterol and 160 μg of budesonide (with each dose consisting of two actuations of 90 μg and 80 μg, respectively [the higher dose combination group]), a fixed-dose combination of 180 μg of albuterol and 80 μg of budesonide (with each dose consisting of two actuations of 90 μg and 40 μg, respectively [the lower-dose combination group]), or 180 μg of albuterol (with each dose consisting of two actuations of 90 μg [the albuterol-alone group]). Children 4 to 11 years of age were randomly assigned to only the lower-dose combination group or the albuterol-alone group.
Results: A total of 3132 patients underwent randomization, among whom 97% were 12 years of age or older. The risk of severe asthma exacerbation was significantly lower, by 26%, in the higher-dose combination group than in the albuterol-alone group (hazard ratio, 0.74; 95% confidence interval [CI], 0.62 to 0.89; P= 0.001). The hazard ratio in the lower-dose combination group, as compared with the albuterol-alone group, was 0.84 (95% CI, 0.71 to 1.00; P = 0.052). The incidence of adverse events was similar in the three trial groups.
Conclusions: The risk of severe asthma exacerbation was shown to be significantly lower with as-needed use of a fixed-dose combination of 180 μg of albuterol and 160 μg of budesonide vs as-needed albuterol alone among patients with uncontrolled moderate to severe asthma who were receiving inhaled glucocorticoid-containing maintenance therapies.
N ENGL J MED 2022: 1-13; DOI: 10.1056/NEJMoa2203163.
Does Tobacco Smoking Influence the Effectiveness of ICS/LABA Controller Therapy in Asthma Patients with Persistent Cold Airway Hyperresponsiveness?
J. M. Perelman1, A. G. Prikhodko2;
Rationale: Smoking in asthma patients can worsen the course of the disease. This study evaluated the effect of the controller therapy with a combination of inhaled corticosteroid/long-acting beta 2-agonist (ICS/LABA) on the clinical course of asthma in patients with persistent cold airway hyperresponsiveness who abuse tobacco.
Methods: The study recruited 84 patients with asthma aged 34 years with persistent cold airway hyperresponsiveness. The 1st group included 44 smokers (5 packs/year), the 2nd group consisted of 40 non-smokers. Asthma control was assessed according to the Asthma Control Test. Lung function before (FEV1,% pred.) and after (ΔFEV1,%) 3-minute isocapnic hyperventilation with cold (-20°C) air (IHCA) was measured. Repeated studies were carried out after 6 and 12 months of ICS/LABA therapy.
Results. Initially, patients in groups 1 and 2 did not have significant differences in ACT (14.9±0.8 vs. 14.6±0.8 points), FEV1 (86.6±2.4 vs. 89.2±2.7%) and ΔFEV1 (-19.1±1.3 vs. -19.4±2.4 %, respectively, p>0.05). In patients of the 1st group, after 6 months, there was a significant increase in ACT to 17.9±1.1 points (p<0.05), an increase in FEV1 to 99.6±3.2% (p<0.01). The airway response to IHCA remained high (ΔFEV1=-19.3±2.9%). After 12 months, ACT in the 1st group became averaged 19.8±1.1 points (p<0.001), FEV1 91.0±3.3%, FEV1=-16.7±2.1%. In patients of the 2nd group, ACT after 6 months was 15.9±1.6 points, after 12 months 18.6±1.7 points (p<0.05). FEV1 at 6 and 12 months was 95.4±2.4 and 95.6±3.8%, respectively (p>0.05). The airway response to IHCA did not change significantly: FEV1 after 6 months was -17.3±1.9%, after 12 months -18.6±3.8% (p>0.05). In the 1st group, there was a correlation between ACT and FEV1 (r=0.35; p<0.05), as well as the smoking index with Tiffeneau index (FEV1/VC, % pred.) (r=-0.39; p<0.001). In the 2nd group, ACT correlated with FEV1/VC,% pred. (r=0.40; p<0.05).
Conclusion. Long-term ICS/LABA controller therapy in smokers and non-smokers with asthma and with persistent cold airway hyperresponsiveness leads to an improvement in lung function, asthma control, however, airway response to a cold stimulus was not attenuated.
A1224; Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.
The Burden of Asthma in Subjects Receiving Low-Dose ICS/LABA - an Analysis from a Real World Survey
K. Gruffydd-Jones1, M. Fagerås2, A. Hamblin3, L. Draffan4, N. Mestdagh5, M. Sidaway6, A. Sheikh7, M. Small8, A. Haq8;
Rationale: A significant number of patients with asthma are maintained on inhaled corticosteroid/long-acting β2-agonist (ICS/LABA) therapy despite continuing to experience symptoms. Healthcare professional (HCP)-perceived adherence to treatment is an important factor in clinical decision-making. This study aimed to understand the burden of disease in subjects with asthma on low-dose ICS/LABA stratified by HCP-perceived adherence.
Methods: Subjects aged ≥18 years with asthma who were on daily low-dose ICS/LABA continuously for 12 months were identified based on information provided by HCPs. Severe asthma exacerbations, disease symptoms in the prior 30 days, rescue inhaler use in the past 4 weeks and HCP-satisfaction with control were assessed for the overall population and by good (‘completely’, ‘very’) or poor (‘moderately’, ‘slightly’, ‘not at all’) HCP-perceived treatment adherence. Analysis was conducted using data from the Adelphi Real World Respiratory Disease Specific Programme (2018-2020), a point-in-time survey conducted in Europe, USA and China.
Results: 79.5% of 497 subjects on daily low-dose ICS/LABA, had good HCP-perceived adherence. Sample sizes may vary between outcomes as not all questions were answered for every subject. Of these subjects, 16.9% experienced ≥1 severe exacerbation in the prior 12 months. Asthma symptoms were reported on 43.0% of the prior 30 days (n=366), and 31.5% used rescue therapy at least once a week (n=349), 27.3% of whom used it over twice-weekly. Subjects experienced disease burden regardless of HCP-perceived adherence, although a numerically higher proportion of subjects with poor HCP-perceived adherence experienced severe exacerbations, symptoms in the prior 30 days and rescue use more than twice a week. Most HCPs perceived their subjects on low-dose ICS/LABA as being at low risk of future exacerbations (91.9%; n=409), although the number of subjects perceived at risk was higher with poor (19.8%) versus good (5.2%) HCP-perceived adherence. HCP satisfaction with asthma control was high (83.7%), and was greater with good (87.3%) versus poor (69.9%) perceived adherence.
Conclusions: Patient management and treatment with current standard of care should be reviewed even when treatment adherence is perceived as good for each child suffering from pediatric asthma as there is residual burden of asthma in patients on low-dose ICS/LABA combination.
A1314; Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.
https://www.abstractsonline.com/pp8/ - !/10476/session/558
Are Patients Treated According to GINA? Treatment and Exacerbation Patterns in UK Primary Care from SABINA Heatmaps
K. Maslova1, J. K. Quint2, M. Fagerås3, A. Baheti4, S. Siddiqui5, R. J. P. van der Valk1;
Rationale: Real-world evidence on asthma treatment practice is limited, however, it could benefit practitioners and healthcare decision makers in aligning treatment with GINA recommendations to reduce the burden of uncontrolled asthma. This study aimed to visualise asthma treatment patterns in relation to exacerbations in UK primary care patients and to quantify the percentage of patients receiving GINA 2016-recommended treatment.
Methods: The study included 189,091 asthma patients (mean age: 45 years, 57.9% female). Individuals ≥12 years with asthma Read codes and research-quality data using UK Clinical Practice Research Datalink (CPRD) primary care GOLD (2008-2019), linked to Hospital Episode Statistics for data on secondary care outcomes, were included in the study. Inhaled corticosteroid (ICS)-containing prescriptions as proportion of days covered and number of short-acting beta-agonists (SABA) canisters were assessed in a 12-month period. Clinical outcomes included exacerbations identified as oral corticosteroid (OCS) bursts, and asthma-related hospitalizations and/or emergency room (ER) visits. Percentage of patients and exacerbation events were quantified for each combination of ICS-coverage (0-100% in 1/12 monthly increments) and SABA prescriptions (0-12+ in 1 canister increments), and visualised in 3D heatmaps using R V4.1.1.
Results: In a 12-month period 5.7% of patients had ≥75% ICS-coverage and <3 SABA and could be considered on GINA 2016 steps 2-5 recommended treatment (Figure 1A); 11.1% of patients were on 0-2 SABA without ICS (GINA 2016 step 1). Almost half of patients (45.7%) had <50% of ICS-coverage and 0-4 SABA (low ICS, low-moderate SABA group), and 13.3% of patients had 12+ SABA across ICS-coverage (high SABA group). These groups accounted for 14.8% (low ICS, low-moderate SABA) and 31.1% (high SABA) of OCS bursts, and 12.7% and 37.8% of hospitalizations and/or ER visits (Figures 1B-1C). Notably, there was a relative absence of hospitalization and/or ER visits (1.9%) among patients with ≥75% ICS-coverage and 0-2 SABA.
Conclusions: 83.2% patients did not receive GINA 2016 steps 1-5 recommended treatment. Many patients use their maintenance irregularly and only in response to seasonal triggers as suggested by the high percentage of patients with low ICS-coverage. Primary care tools to identify patients with low ICS and excessive SABA prescriptions are needed. Care-based interventions are required to optimise asthma management of patients with 12+ SABA canisters/year.
A2125; Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.
Critical Errors in Inhaler Technique in Patients with Pediatric Asthma and Risk of Hospital Admissions: Socio-Ecological Study from Eastern Nepal
N. K. Bhatta, H. Prasad, S. Chaudhary, L. Sah; Pediatrics, BPKIHS, Dharan, Nepal.
Introduction and Rationale: The connection between socio-demographic characteristics, medication compliance, and hospitalisation risk in paediatric asthma is investigated using a socio-ecological study design. Children who make crucial inhaler technique errors are at a higher risk of hospitalisation. Despite the global importance of this issue, there is a lack of data on significant inhaler technique errors and their association to the risk of hospital admissions for asthma in Nepalese children. In this context, we conducted a hospital-based cross-sectional study using a socio-ecological model to identify key errors in inhaler technique in children with asthma and investigate their link with the probability of hospital admissions.
Methods: This cross-sectional study was undertaken in the Department of Pediatrics at B. P. Koirala Institute of Health Science (BPKIHS), a university teaching hospital in Nepal, on all children diagnosed with asthma aged 5-14 years who were admitted to hospital between June 2017 and May 2018. Detailed socio-demographics, asthma clinical characteristics and severity, and asthma medication usage history were also recorded. They were instructed to use their inhaler device as usual, with technique evaluated using the Inhaler Device Assessment Tool (IDAT) and asthma control evaluated using the A-CAT. An action or inaction that might have a definite negative influence on drug delivery to the lungs was characterised as a critical inhaler mistake. Critical flaws in inhaler technique were discovered within a socio-ecological framework. To explore their relationships with risk of hospital admissions, descriptive and inferential statistics were used
Results: 100 children with asthma were enrolled in the study. The age range was 4-11 yrs; (Mean age was 6.28 year) and 70 % boys. (40%) of them were hospitalized. Among them 50% had critical errors in inhaler technique; The most common was blocking the spray with teeth or tongue (40%), not shaking the inhaler before use (30%) and wrong breathing techniques (30%). Parents of (40%) children had incorrect perceptions about asthma. Children using 2 inhaler device, having parents of low SES and with infrequent OPD follow-up were twice likely to make ≥1 critical error and being hospitalized.
Conclusions: Hospitalization of substantial proportion of children with asthma is attributed to critical error in inhaler technique. Pediatricians in Nepal must play a more proactive rather reactive role in teaching the correct inhaler technique, assessing inhaler technique and choosing an optimal inhaler device individually for each child suffering from pediatric asthma.
A1791; Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.
Prediction Score for the Diagnosis of Asthma with Respiratory Symptoms: PIFA Score (Provocation Test, Induced Sputum Eosinophilia, and FeNO for Diagnosing Asthma)
This study aimed to develop a multivariable scoring model based on objective tests, by stratifying the risk of patients with asthma from patients without asthma to predict the presence of asthma in patients with respiratory symptoms, Treatment-naïve adult subjects with respiratory symptoms (cough, dyspnea or wheezing) undergoing bronchial challenge test with methacholine, FeNO and induced sputum eosinophil count (ISE) for suspicion of asthma were reviewed. A new scoring system for predicting the diagnosis of asthma was developed using beta coefficients of the final multiple regression models. Of 760 subjects, 302 patients (39.7%) were diagnosed as asthma. The subjects with a PIFA score of 5-point or greater were likely to have asthma with sensitivity 79.7%; specificity 92.7% and accuracy of 91%. The PIFA score showed a good discriminative ability for the diagnosis of adult asthma. The authors of the study called for further research to validate this novel scoring system.
A1241; Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.
Effect of the COVID-19 Pandemic on Asthma Morbidity Among Children Hospitalized for an Exacerbation
Background: Although COVID-19 pandemic was associated with a remarkable decrease in pediatric asthma ED visits and hospitalizations, there is limited information on the clinical characteristics of children hospitalized for asthma during the pandemic.
Method: This retrospective study conducted at Children's National Hospital, Washington, DC, compared demographic and clinical characteristics of children, 2-18 years old, hospitalized for an asthma exacerbation between April-September 2020 (cases) to April-September 2019 (controls).
Result: Data from the study showed that children hospitalized during the pandemic were older than those hospitalized the prior year (9.85 ±4.35 vs. 6.68 ± 3.82, P <.0001). Magnesium sulfate was more frequently administered to the cases than to the controls (84% vs. 63%, P=0.0041), and its use was independent of differences in age and comorbid conditions. Although a higher ratio of cases was non-compliant with preventative medications (46% vs. 24.7%, P=0.0023), a lower proportion of them were kept on inhaled corticosteroids during the hospital stay (30% vs. 58.8%, P= 0.0002).
Conclusion: The study corroborated a significant drop in pediatric asthma hospital admissions during the COVID-19 pandemic, but reported higher use of magnesium sulfate in the ED among children hospitalized during the pandemic, suggestive of more severe presentation of pediatric asthma exacerbations during the pandemic compared to the prior year.
A1170; Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.
Efficacy and Safety of Albuterol/Budesonide in Mild-to-Moderate Asthma: Results of the DENALI Study
Rationale: Short-acting β2-agonists provide quick asthma symptom relief but fail to address underlying inflammation. Combining albuterol and budesonide, an inhaled corticosteroid, in a single inhaler as rescue therapy for asthma could provide rapid bronchodilation while treating airway inflammation.
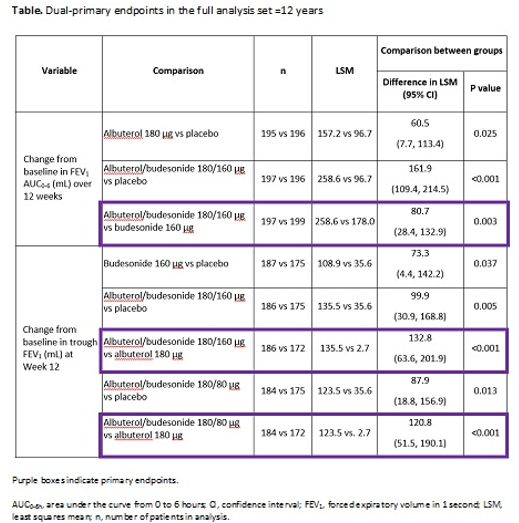
Methods: The Phase 3 DENALI study (NCT03847896) evaluated contributions of the mono-components to albuterol/budesonide efficacy in patients with mild-to-moderate asthma ≥4 years. Patients ≥12 years were randomized 1:1:1:1:1 to four-times-daily albuterol/budesonide 180/160 or 180/80 μg, albuterol 180 μg, budesonide 160 μg or placebo for 12 weeks; patients 4-11 years were not included in this analysis set. Dual-primary endpoints were change from baseline in forced expiratory volume in 1 second area under the curve from 0-6 hours (FEV1, AUC0-6h) over 12 weeks and in trough FEV1 at Week 12. Secondary endpoints included time to onset, as defined by a 15% improvement in FEV1 within 30 minutes on Day 1, and duration of effect, and Asthma Control
Questionnaire-7 (ACQ-7) responder (≥0.5-point reduction from baseline) analysis at Week 12.
Results: Of 1001 patients randomized, 989 were aged ≥12 years (mean age 48.9 years, 62.2% female). Change from baseline in FEV1 AUC0-6h over 12 weeks was greater with albuterol/budesonide 180/160 μg (only dose tested) versus budesonide (least squares mean [LSM] difference 80.7 mL, 95% confidence interval [CI] 28.4-132.9; p=0.003; Table). Change in trough FEV1 at Week 12 was greater with albuterol/budesonide 180/160 and 180/80 μg versus albuterol (LSM difference 132.8 mL [95% CI 63.6-201.9] and 120.8 mL [95% CI 51.5-190.1], respectively; both p<0.001; Table). Median time to onset and duration of effect on Day 1 (responders: 49.7%, 44.0% and 42.9%), was 7.5 and 7.0 minutes vs 9.5 minutes, and 186.9 and 191.4 minutes vs 168.2 minutes, respectively, for albuterol/budesonide 180/160 or 180/80 μg and albuterol. At Week 12, percentages of ACQ-7 responders were 66.5% and 65.5% vs 47.2% with albuterol/budesonide 180/160 or 180/80 μg vs albuterol (odds ratio [OR] 2.3 [95% CI 1.5-3.7] and OR 2.3 [95% CI 1.5-3.6]; both nominally significant), respectively. The safety profiles for both albuterol/budesonide doses were similar to the mono-components.
Conclusions: Both mono-components contributed to albuterol/budesonide efficacy, with the combinations demonstrating superior effects on lung function. Onset and duration of bronchodilation were similar for albuterol/budesonide vs albuterol on Day 1, and more patients experienced a nominal improvement in asthma control at Week 12. The overall clinical profile of albuterol/budesonide observed supports its potential utility as a future novel rescue therapy.
A3414; Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.
Idiopathic Pulmonary Fibrosis
Impact of Comorbidities in Idiopathic Pulmonary Fibrosis Patients: Based on Nationwide Claim Data
J. Lee, L. Hoon Hee, P. Hyung Jun, K. Ho Cheol.
Background: Comorbidities in idiopathic pulmonary fibrosis (IPF) affect quality of life, symptoms, disease progression and survival.
Objective: To investigate prevalence, incidence, and clinical impact of comorbidity in IPF patients, based on nationwide claim data in South Korea.
Methods: Nationwide health claim data between 2011 and 2019 were collected from the Korean Health Insurance Review and Assessment (HIRA) database. The presence of the comorbidities was defined by at least one claim of disease-specific ICD-10 codes. Burden of comorbidities was presented using Charlson comorbidity index (CCI).
The patients were categorized into two groups according to pirfenidone use (pirfenidone user group vs. non-user group), used dose of pirfenidone (standard-dose group vs. low-dose group), and CCI (high CCI group vs. low CCI group).
Results: Between 2011 and 2019, prevalence and incidence rate increased annually from 7.50 to 23.20 and from 3.56 to 7.91 per 100,000 patients, respectively in the Korean population.The most common respiratory comorbidity was chronic obstructive pulmonary disease (COPD, 37.34%), followed by pulmonary tuberculosis (5.24%), and lung cancer (3.34%). Gastroesophageal reflux disease (70.83%), dyslipidemia (62.93%), and hypertension (59.04%) affected more than half of total IPF patients. Prevalence of pulmonary hypertension, pulmonary embolism, pulmonary tuberculosis, and non-tuberculosis mycobacteria pulmonary disease were higher in the non-user group. In non-respiratory comorbidities, prevalence of hypertension, depression, anxiety and congestive heart failure was higher in the non-user group and the low dose group.Compared with the standard-dose user group, COPD more common in the low-dose group. CCI was significantly higher in male and old age group. Male, young age and patients with low CCI were more common in pirfenidone user group. High CCI group used more medical resource related to admission. Otherwise, low CCI group spend more medical costs associated with outpatient clinics. Total medical cost was higher in high CCI group.
Conclusion: Prevalence of comorbidities were variable based on the clinical characteristics in IPF patients. Several comorbidities and disease burden in IPF patients were associated with the use of pirfenidone and increased medical costs.
A3528; Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.
Elevated Neutrophil and Monocyte Counts Can Predict Acute Exacerbations in IPF Patients
N.Arai. Respiratory Medicine, Ibarakihigashi National Hospital, Naka-gun, Japan.
Background: Nintedanib is now standard of care for the treatment of idiopathic pulmonary fibrosis (IPF). Nintedanib suppresses various fibrotic processes in IPF and reduces the rate of decline in forced vital capacity (FVC). However, the host factors that predict the efficacy of nintedanib is not known.
Objective: To investigate the effects of nintedanib in IPF patients by using clinical information, and to examine the host factors that predict the therapeutic effect of nintedanib to IPF patients.
Methods : IPF patients treated with nintedanib between March 2019 and May 2021 were enrolled in the study. The changes in FVC, % FVC, diffusing capacity of the lung for carbon monoxide (DLCO), % DLCO, LDH, Krebs von den Lungen-6 (KL-6), surfactant protein-D (SP-D), and modified Medical Research Council (mMRC) were evaluated.
Patients with ≥10% decline in FVC or ≥ 15% decline in DLCO from baseline to 6 months after treatment were classified as the non-responder group, while the other patients were classified as the responder group. In addition, patients who experience acute exacerbation despite nintedanib treatment were defined as the acute exacerbation group.
Results: Out of 87 IPF patients who were evaluated 64% were classified as responders; 25% were classified as non-responder group and 10% as acute exacerbation group. Non-responders showed a significantly lower body mass index (BMI) (22.9 p = 0.029) as compared to responders. The acute exacerbation group also had a significantly lower BMI (21.4 , p = 0.023) and %DLCO(34.8, p = 0.039) at baseline as compared to the non-acute exacerbation group. The number of peripheral neutrophils and peripheral monocytes were significantly higher in the acute exacerbation group than those in the non-acute exacerbation group. At the time of acute exacerbation, the serum LDH level increased significantly (p = 0.008) as compared to baseline.
Conclusions: Low BMI at baseline may be useful in predicting a nintedanib response in IPF patients. Elevated neutrophil and monocyte counts can be predictors of acute exacerbations.
A2431; Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.
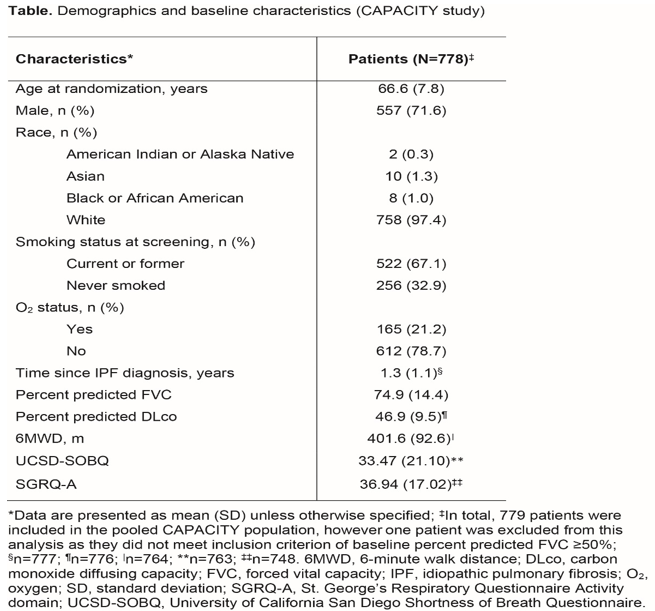
Smaller Deterioration in UCSD-SOBQ Scores May Be Clinically Meaningful in Patients with IPF with Less Impaired Lung Function: Findings from The CAPACITY Studies
K. I. Aronson, G. Raghu, S. Gupta, J. Ko, D. Kile, E. Neuberger, J. Devine, J. J. Swigris;
Background: The UCSD-SOBQ is a 24-item patient-reported measure of dyspnea (range 0-120; higher score indicating greater dyspnea). In STEP-IPF study that was conducted in advanced IPF patients, 24-week UCSD-SOBQ change scores were anchored to the following measures of worsening: ≥7% decline in raw forced vital capacity (FVC); ≥15% decline in raw carbon monoxide diffusing capacity (DLco); ≥20% (or ≥30 m) decline in 6-minute walk distance (6MWD); and ≥5 points increase in the St. George’s Respiratory Questionnaire Activity domain (SGRQ-A) score. The MCID in UCSD-SOBQ scores was estimated as 8 (range 5-11).
Objective: To evaluate a similar threshold in patients with IPF with less impaired lung function over 72 weeks.
Method: Data were pooled from the CAPACITY trial which included patients with baseline percent predicted FVC ≥50%. Baseline characteristics were described, correlations between UCSD-SOBQ scores and potential anchors were evaluated at baseline, and MCIDs were estimated using anchor- and distribution-based methods. For anchor-based methods, the optimal cut-point was identified by Youden index analysis from the receiver operating characteristic curve. Cronbach’s alpha standard error of measurement and Cohen’s moderate effect size were estimated as the distribution-based methods.
In this analysis, change from baseline to Week 72 in UCSD-SOBQ scores were anchored to the following measures of worsening: ≥5% decline in raw FVC; ≥10% decline in raw DLco; ≥24 m decline in 6MWD; and ≥5 points increase in SGRQ-A.
Result: Table 1 represent the baseline characteristicsAt baseline, UCSD-SOBQ scores correlated- weakly with FVC (correlation coefficient: -0.2225) and DLco (-0.2479); moderately with 6MWD (-0.3347); and strongly with the SGRQ-A score (0.8054).The optimal MCID cut-points for UCSD-SOBQ at Week 72 were estimated as 6 for FVC, 9 for DLco, 4.3 for 6MWD, and 5.8 for SGRQ-A. Distribution-based results were supportive in interpreting the anchor-based results.
Conclusion: Overall, the results suggest a MCID range of 4-6, with the DLco anchor excluded due to the limited use of DLco clinically as a marker of disease progression in IPF. These findings suggest that deterioration in UCSD-SOBQ scores smaller than previously reported may be clinically meaningful in patients with IPF with less impaired lung function.
A3535; Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.
Early Intervention with Sildenafil Plus Pirfenidone in Patients with Advanced Idiopathic Pulmonary Fibrosis and Risk of Pulmonary Hypertension (PH): Results of A Responder Analysis
S. Harari, S. D. Nathan, J. Behr, W. A. Wuyts, K. Kirchgaessler, M. Bengus, F. Gilberg, A. U. Wells;
Background: STEP-IPF (NCT02951429) trial failed to demonstrate a treatment benefit of sildenafil in addition to pirfenidone.
Objective: This was post hoc analysis of STEP-IPF study to understand responder analysis at 6 and 12 months of therapy.
Method: For this analysis, responder was defined as patients who did not progress; specifically, those that were alive with no respiratory-related non-elective hospitalization, no disease progression (<15% 6MWD decline, <5% absolute FVC (% predicted) decline, and <4-point increase in SGRQ total score) and no discontinuation due to an adverse event (AE). The proportion of responders was compared using the McNemar test for the comparison of time points within treatment groups.
Results: 177 patients with advanced IPF and risk of Group 3 PH were randomized. The proportion of responders at 6 months was 63.6% in the pirfenidone plus sildenafil, and 55.1% in the pirfenidone plus placebo group; at 12 months, the respective responder rates were 36.4% and 33.7%. The differences within groups between month 6 and month 12 were statistically significant (p<0.001). The only major differences in baseline demographics of the responder vs non-responder groups were lower median DLco (30.0 and 30.1% vs 25.1 and 24.0%) and a higher median SGRQ total score (56.4 and 54.9 vs 61.9 and 63.8).
Conclusion: Adding sildenafil to pirfenidone resulted in a numerically higher proportion of responders at 6, but not at 12 months of treatment. Non-responders had lower DLco and worse quality of life at baseline, suggesting that earlier intervention might be more promising while remaining of a transient nature.
A; Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.
Combined Assessment of the GAP Index and Body Mass Index at Antifibrotic Therapy Initiation for Prognosis of Idiopathic Pulmonary Fibrosis
Y. Suzuki, K. Mori, M. Kono, H. Hasegawa, K. Yokomura, H. Hozumi, M. Karayama, K. Furuhashi, N. Enomoto, T. Fujisawa, Y. Nakamura
Background: Both nintedanib and pirfenidone have shown to slow down the disease progression in IPF progressive fibrotic interstitial lung disease patients by reducing the annual decline in forced vital capacity. Recent advances have revealed the importance of body-composition factors in the prognosis of IPF treated with antifibrotics.
Objective: This study aimed to evaluate the GAP index and the involvement of the body mass index (BMI) at the time of antifibrotic initiation for predicting the prognosis in IPF patients.
Methods: This was a multi-centre, retrospective study that included IPF receiving antifibrotic and were divided into two cohorts i.e., the Hamamatsu cohort (n=110) and Seirei cohort (n=119).
Results: The distribution of GAP stages I, II, and III was 38.2%, 43.6%, and 18.2%, respectively, in the Hamamatsu cohort and 41.2%, 50.4%, and 8.4%, respectively in the Seirei cohort. In both cohorts, the GAP index distinctly classified the prognosis into three groups as shown in figure below. A lower BMI showed prognostic value independent of the GAP index in multivariate analyses. Combining the GAP index with the BMI at antifibrotic initiation successfully divided the patients with IPF into four distinct prognoses.
Conclusion: The present study showed that assessment of the GAP index at antifibrotic initiation distinctly classified the IPF prognosis. Additionally, combining the GAP index with BMI successfully divided patients with IPF into four distinct prognoses, suggesting the importance of the GAP index and BMI for prognostic prediction at antifibrotic initiation in IPF.
A2430; Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.
Pulmonary Hypertension
Sildenafil Added to Pirfenidone in Patients with Advanced Idiopathic Pulmonary Fibrosis and Risk of Pulmonary Hypertension (PH): Improves on Health-Related Quality of Life
W. A. Wuyts, S. D. Nathan, A. U. Wells, S. A. Harari, K. Samara, M. Bengus, F. Gilberg, J. Behr
Background: SP-IPF (NCT02951429) was a Phase 2b double-blind, randomised, placebo-controlled study of the efficacy and safety of sildenafil added to pirfenidone in patients with advanced idiopathic pulmonary fibrosis (IPF) at risk of Group 3 pulmonary hypertension (PH) based on right heart catheterization (RHC) or Echocardiography (ECHO) criteria. The primary analysis in that study was percentage of patients with disease progression, defined as a 6-minute walk distance decline, respiratory-related hospitalization or all-cause mortality over 52 weeks who failed to demonstrate a treatment benefit of sildenafil in addition to pirfenidone.
Objective: The present study represents the post hoc analysis conducted to evaluate the impact of health-related quality of life as measured by the SGRQ.
Methods: In total, 177 patients with advanced IPF and risk of Group 3 PH were randomized. The proportion of patients who showed an improvement of more than 4 points (responders) in the total SGRQ score at any time during the study and the corresponding risk difference (exact method) using a one-sided Chi-square test was analyzed. This cut-off corresponded to the MCID previously established in the STEP-IPF study population. The mean change in total SGRQ score was compared using rank ANCOVA testing as well as the Hodges-Lehman (HL) median difference in change in total score in the responder groups.
Results: The proportion of patients with a >4-point improvement in total SGRQ score over the 52-week study period was 39.8% in the sildenafil plus pirfenidone group (N=35 of N=88) and 27.0% (N=24 of N=89) in the placebo plus pirfenidone group (risk difference 16.69%, 95% CI -0.35; 32.86; p=0.0486). In the responder groups, the difference between treatment groups in mean change in total score was -7.92 points (rank ANCOVA p=0.3679), and the HL median difference in change was -5.42 points (95% CI -18.53; 4.85).
Conclusions: There was a significantly higher proportion of patients in the sildenafil plus pirfenidone group showing an improvement in health-related quality of life exceeding the MCID established in advanced IPF. In the responder groups, the changes in SGRQ total scores over the 52-week period were numerically in favor of sildenafil plus pirfenidone. Although exploratory, these findings are relevant when considering treatment with sildenafil in patients with advanced IPF and risk of PH.
A3533; Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.
Effectiveness of Inhaled Treprostinil in Patients with Group 3 Pulmonary Hypertension
M. Mohamed, S. Shaikh, J. K. Mwangi, S. Patolia.
Background: Pulmonary hypertension (PH) remains a major cause of morbidity and mortality, with limited pharmacological options. PH due to lung disease classified as group 3 by World Health Organization (WHO) has been reported in about 86% of patients with interstitial lung disease (ILD). Though vasodilators have become standard of care for treatment of pulmonary arterial hypertension (PAH), there was approved treatment for Group 3 PH.
Objective: Conduct systematic review and meta-analysis to evaluate the potential role of inhaled treprostinil in patients with group 3 PH.
Methods: Multiple data bases using prespecified search terms were screened and the analysis included only those patients with pulmonary hypertension (confirmed by right heart catheterization) caused by intrinsic lung disease. Longitudinal studies comparing inhaled treprostinil to standard of care were eligible.
Primary outcome of interest was change in 6-minute walk distance (6MWD), while changes in FVC%, SPO2%, and St. George’s Respiratory Questionnaire (SGRQ) were measured as secondary outcomes.
Results: A total of 4 studies were included (one randomized trial and three retrospective cohorts ) with a total of 392 patients and a median (range) follow up of 16 (12-24) weeks. The mean age of patients was 63.1±10.6 years and about 54% were men. At the baseline, mean pulmonary artery pressure (mPAP) was 42.5±8.4 mmHg, pulmonary vascular resistance (PVR) was 8.4±2.8 wood unit (WU), and 6MWD was 268.6±85 m. 70% of the patients had restrictive lung disease. Patients who received inhaled treprostinil had a significant increase in 6MWD [MD 32.52, 95%CI (19.32,45.72), P<0.01] compared to standard of care. However, there was no significant difference between inhaled treprostinil or standard of care regarding changes in FVC%, SPO2%, and SGRQ [MD 1.0, 95%CI (-2.09,4.10), P=0.53], [MD -0.19, 95%CI (-1.63,1.24), P=0.79], [MD -0.35, 95%CI (-5.18,4.48), P=0.89] respectively.The most common AE was reported with inhaled treprostinil was cough and headache. No significant difference between groups regarding severe AE.
Conclusion: Inhaled treprostinil is associated with a significant increase in 6 MWD compared to standard of care in patients with PH due to intrinsic lung disease. Larger, randomized studies are awaited.
A;Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.
Hypersensitivity Pneumonitis
Foam Mattress – A Novel Source of Trigger for Hypersensitivity Pneumonitis
S. Tang, O. Moran Mendoza; Medicine, Queen's University, Kingston, ON, Canada.
Background: Hypersensitivity pneumonitis (HP) is type of interstitial lung disease (ILD) resulting from immune-mediated reactions from inhaled antigens. Though many causal agents have been identified, up to 60% of patients do not have an identifiable trigger.
Objective: To report a case of HP associated with exposure to mould in foam from bedding.
Result: A 76-year-old man presents to ILD clinic with gradually worsening dyspnoea (MRC 2/5) and dry cough over several years. He had no infectious or constitutional symptoms. He had a remote 8-pack-year smoking history and retired as a mechanic 15 years prior.
The current CT chest showed upper lobe predominant reticulation, traction bronchiectasis and honeycombing in keeping with an indeterminate usual interstitial pneumonia pattern. There was no history of exposure to birds, moulds, chemicals, fumes, or drugs associated with ILD, and no clinical or serologic evidence of connective tissue diseases. However, he reported having foam pillows and mattress, and occasional exposure to wood dust. His past medical history was relevant for mild dementia, remote peptic ulcer and bladder surgeries.
All his pulmonary function tests findings i.e., FVC 90%, FEV1 115%, FEV1/FVC 84%, TLC 84%, DLCO 101% were normal. There were no major findings from bronchoalveolar lavage (lymphocytes 6%, neutrophils 2%, macrophages 92%). VATS biopsy showed granulomatous ILD compatible with HP. On multidisciplinary discussion, clinician came to a diagnosis of HP most likely related to exposures to foam in his pillows and mattress, and less likely to occasional wood dust exposure. Post the avoidance of such exposures his respiratory function stabilized for 2.5 years, though he developed worsening dyspnea (MRC 3/5), DLCO (63%) and TLC (65%).
During home visit, it was notice that below an uncovered foam in his mattress grew Penicillium sp. and Aspergillus sp. Later there was improvement in his symptoms after moving to his daughter’s house and a course of dexamethasone. However, when he returned to his home worsening of dyspnea progressed (MRC 5/5) was reported despite removal of the mattress. A video home visit found foam cushions below his recliner and desk chair, where he spent significant time and shortly thereafter, he died of an ILD exacerbation.
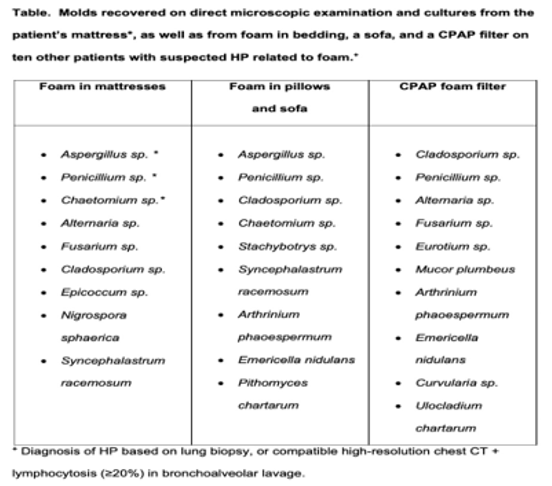
Conclusion: Foam is ubiquitous in households. This study found that moulds that cause HP in various foam sources (Table) in patients diagnosed with HP. Foam in bedding and other sources is a novel vector of moulds that may explain a large proportion of HP patients with no prior identified trigger. Seeking and removing foam from patients’ environments could prevent HP progression to end-stage disease and death.
A1111; Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.
COVID-19
Prevalence and Mechanisms of Bronchiolar Mucus Plugging in COVID-19 Lung Disease
T. Kato1, T. Asakura1, C. E. Edwards2, H. Dang1, Y. Mikami1, K. Okuda1, G. Chen1, L. Sun1, R. C.
Rationale: A comprehensive characterization of mucus obstruction despite reports of excessive mucus in coronavirus disease 2019 (COVID-19) has not been reported. The frequency of airways mucus plugging, pattern of mucin hypersecretion, and molecular regulation of mucin expression in COVID-19 lung disease, needs to be evaluated.
Methods: The autopsy of COVID-19 lungs (N=61 subjects) was performed for evaluating airway mucus and mucins by Alcian blue and periodic acid-Schiff (AB-PAS) staining and RNA in situ hybridization for MUC5B, MUC5AC and additional mucin-related genes. To investigate mechanisms of SARS-CoV-2-induced mucin secretion and to test candidate countermeasures by bulk RNA-sequencing, AB-PAS staining, and immunohistochemistry (IHC), SARS-CoV-2-infected human bronchial epithelial (HBE) cultures (N=14 donors) were utilized. To investigate molecular pathways responsible for mucin induction following SARS-CoV-2 infection, Epidermal growth factor receptor (EGFR) and interleukin-1 receptor (IL-1R) pathways were blocked in HBE cultures (N=6 or 7 donors/condition) by inhibitors and/or CRISPR/Cas9-mediated gene deletion.
Results: Typically, in the sub-acute/chronic disease phase following SARS-CoV-2 viral clearance, MUC5B and MUC5AC RNA and protein levels were increased in COVID-19 autopsy tracheas. In the distal lung, MUC5B-dominated mucus plugging was frequently observed in bronchioles (>90% of COVID-19 subjects), and MUC5B accumulated in alveolar regions. MUC5B was secreted from bronchiolar and not alveolar cells as it was suggested by molecular studies. At day 3 post inoculation (pi), SARS-CoV-2 titers peaked in HBE cultures. Induction of goblet cell metaplasia and MUC5B and MUC5AC RNA/protein peaked at day 7-14 pi. SARS-CoV-2 infection of HBE cultures induced expression of EGFR ligands and inflammatory cytokines associated with mucin gene regulation, e.g., IL-1α/β. Inhibiting EGFR or IL-1R pathways reduced mucin expression following SARS-CoV-2 infection.
Conclusion: In COVID-19 autopsy lungs, SARS-CoV-2 infection is linked to a significant frequency of bronchiolar mucus blockage. In the sub-acute and chronic phases of SARS-CoV-2 infection, HBE experiments identified roles for EGFR and IL-1R signalling in mucin gene regulation. These findings imply that treating COVID-19 lung illness with time-sensitive administration of mucolytic drugs and/or particular pathway inhibitors could be beneficial.
A3612; Presented at American Thoracic Society (ATS), 13-18th May 22002, San Francisco, CA.